Lung Cancer Choices© 6th Edition Menu
Chapter 1: Diagnosis and Staging of Lung Cancer
Chapter 2: Comprehensive Biomarker Testing
Chapter 3: Surgery for Lung Cancer Patients
Chapter 5: Radiation Therapy for Non-Small Cell Lung Cancer
Chapter 6: Treatment for Small Cell Lung Cancer
Chapter 7: Clinical Trials and Emerging Therapies for Lung Cancer
Chapter 9: Nutrition in the Patient with Lung Cancer
Chapter 10: Sexuality and Lung Cancer
Chapter 11: Integrative Medicine, Complementary Therapies, and Chinese Medicine in Lung Cancer
Chapter 12: Lung Cancer in People who have Never Smoked
Chapter 13: How to Quit Smoking Confidently and Successfully
Lung Cancer Question Builder - Develop your list of "Questions to Ask" your providers
Clinical Trials and Emerging Therapies for Lung Cancer
Emily Duffield, MPH, MSN, ANP-BC
Introduction
Clinical research continues to identify and develop novel treatment options for lung cancer patients. Multiple new treatments have been made commercially available over the past few years, while others still in the developmental pipeline remain available only to patients who participate in clinical trials. There are several different classes of systemic therapy used to treat lung cancer, including chemotherapy, targeted therapy, and immunotherapy. Although traditional chemotherapy remains an important tool for lung cancer treatment, both targeted therapies and immunotherapies are playing an increasingly important role in treatment, particularly for patients diagnosed with advanced stages of the disease.
Instead of attacking all rapidly dividing cells the way chemotherapy does, targeted therapies concentrate on specific genetic abnormalities unique to some tumors, often resulting in fewer side effects and the potential for improved cancer control. Targeted therapies can pinpoint specific DNA mutations in the tumor and utilize them to prevent cancer cells from growing and dividing out of control. Targeted therapies may be used alone or in combination with other treatments to improve overall survival. The drawback to targeted therapies is that they can only be utilized by a select group of patients who have tumors with unique DNA mutations. As a result, these therapies are not indicated for all patients.
 Immunotherapy is a newer type of targeted therapy that is appropriate for many lung cancer patients. Immunotherapy medications interact with certain receptors on tumor cells and immune cells to change the way the body reacts to a tumor, often allowing the immune system to recognize and attack tumors. In some cases, turning on the immune system with the help of immunotherapy can even cause the body to completely eliminate the tumor. Immunotherapies are showing great promise in lung cancer as well as in many other types of advanced malignancies.
Immunotherapy is a newer type of targeted therapy that is appropriate for many lung cancer patients. Immunotherapy medications interact with certain receptors on tumor cells and immune cells to change the way the body reacts to a tumor, often allowing the immune system to recognize and attack tumors. In some cases, turning on the immune system with the help of immunotherapy can even cause the body to completely eliminate the tumor. Immunotherapies are showing great promise in lung cancer as well as in many other types of advanced malignancies.
While many of the recently approved therapies appear to be very promising, chemotherapy remains an important part of the treatment plan for many lung cancer patients, and researchers continue to evaluate the effect of combining chemotherapy with immunotherapies and targeted therapies in an effort to further improve outcomes for patients diagnosed with lung cancer. Other areas of research include the utilization of targeted agents and chemotherapy for lung cancer maintenance therapy (prevention of relapse) and medications to prevent lung cancer (chemoprevention) in patients at high risk for developing this disease. The recently approved targeted and immunotherapies are already improving progression-free and overall survival rates for lung cancer patients, but there is more work to be done. Research and clinical trials continue to evaluate the best use of novel therapeutic agents to improve the quality of life and longevity of patients with lung cancer.
Clinical Trials
Drug development begins with the identification of new substances that show anti-cancer activity in research laboratories. Following extensive laboratory testing, clinical trials are done to establish whether these substances are safe and effective at fighting cancer in people. The purpose of clinical trials is to identify new agents that can improve survival or quality of life compared to currently available treatments.
Clinical trials of new drugs are done in a series of phases, each with a specific purpose. If the drug is safe and provides benefit in an early phase trial, it is further tested in subsequent phases:

- Phase 1: the drug is tested for the first time in people to establish safety, tolerability, dosage, and treatment schedule for subsequent studies.
- Phase 2: the drug is tested in a larger group of people to continue to evaluate efficacy and safety, and to identify the range and severity of side effects.
- Phase 3: the drug is tested in an even larger group of people to determine whether the new drug is more effective than existing treatments. Side effects and safety are also monitored. FDA approval for drugs is typically based on the results of Phase 3 trial data.
- Phase 4: after approval by the United States Food and Drug Administration (FDA), the drug is available for use in the general population and further monitored for safety, efficacy, and long-term side effects.
During Phases 1 to 3, the drugs are available only to patients who participate in the clinical trial. In phase 4, the drugs are commercially available through drug stores and specialty pharmacies. Clinical trials have historically been available only at major medical centers but are increasingly becoming available at smaller community medical centers due to the expansion of hospital networks. A list of all clinical trials available for lung cancer patients is provided on the Internet site of the National Cancer Institute (http://www.cancer.gov/clinicaltrials/search). The treating oncologist may recommend trials that are available locally as well as at regional medical centers.
Sometimes new drugs that demonstrate a major increase in efficacy compared with older therapies are granted FDA Breakthrough Therapy or Fast Track status. The status expedites the development and FDA review process with the goal of making these exciting new treatments available to patients in the shortest possible time while still preserving the research process and maintaining patient safety. Drugs with Fast Track Status or Breakthrough Therapy designation are initially available only in clinical trials, but typically move through the clinical trial process and become widely available through commercial dispensing pharmacies much more quickly than if they had followed traditional approval pathways. Certain targeted therapies have shown such improvement in efficacy and tolerability compared to standard therapies that they have moved from Phase 1 “first in human trials” to FDA approval in under four years. Shortening the timeline for moving novel drugs from the laboratory into the clinic for patient use has clear benefit for improving patient outcomes, particularly in lung cancer where for many years, there has been a clearly documented need for new and improved treatments.
Targeted Therapy
 Chemotherapy drugs work by killing cancer cells that multiply rapidly. However, many normal cells also multiply rapidly, such as cells of the digestive tract, hair follicles, and blood. When these normal cells are affected by chemotherapy drugs, undesirable side effects occur. Targeted therapy includes newer drugs that interfere with specific aspects of cancer cells, minimizing damage to normal cells. Targeted therapy consists of either monoclonal antibodies (drug names ending in “-ab”) that target the outside surface of the cancer cell or small molecules (drug names ending in “-ib”) that target the inside of the cancer cell.
Chemotherapy drugs work by killing cancer cells that multiply rapidly. However, many normal cells also multiply rapidly, such as cells of the digestive tract, hair follicles, and blood. When these normal cells are affected by chemotherapy drugs, undesirable side effects occur. Targeted therapy includes newer drugs that interfere with specific aspects of cancer cells, minimizing damage to normal cells. Targeted therapy consists of either monoclonal antibodies (drug names ending in “-ab”) that target the outside surface of the cancer cell or small molecules (drug names ending in “-ib”) that target the inside of the cancer cell.
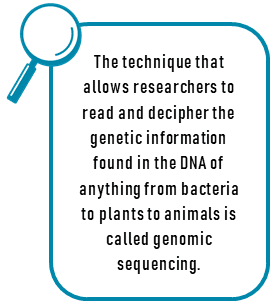
As genetic research advances, great strides are being taken to better understand the molecular make-up of tumors, and to determine the mechanisms which drive tumor development, growth, and spread to other organs (metastasis). The wider availability of full genome sequencing of tumor DNA is opening up the opportunity for truly personalized medicine, in which therapies are targeted to the specific genetic make-up of an individuals’ tumor. Genome sequencing offers the opportunity to identify rare mutations and then design a treatment plan to block the exact mechanism that is making the cancer grow. Examples of well-studied mutations that are common in lung cancer are EGFR mutations, EML4-ALK gene rearrangements, and KRAS mutations. Newer targets being researched in lung cancer include ROS1, BRAF, HER2, MET, PIK3CA, RET, MEK, and NTRK. Several drugs used to target these mutations have been approved by the FDA for either lung cancer or other types of cancer, while many of the novel agents listed below are available only through clinical trials.
Monoclonal Antibodies
Monoclonal antibodies are one type of signaling molecule that binds to receptors on the cell surface. When these signaling molecules stimulate cell surface receptors, they initiate a cascade of messages inside the cell that promotes cellular growth and development. The normal cellular controls for this process are absent in malignant cells, and cellular replication proceeds uncontrolled. Antibodies are produced by the immune system to fight infections caused by bacteria or viruses, and the body produces specific antibodies for each type of infectious agent (antigen) to which the body is exposed. Identification of tumor-specific antigens allows novel drugs to use the immune response to recognize and fight cancer cells. This class of drugs, known as monoclonal antibodies, are produced in a laboratory and are designed to bind with a very specific target, such as a cell surface receptor or other defects unique to cancer cells.
Monoclonal antibodies fight cancer cells through several mechanisms, including:
-
- Blocking cell surface receptors to turn off the downstream cell signaling cascade.
- Targeting specific defects in the cancer cells or labeling the cancer cells, making them more vulnerable to destruction by the body’s immune system.
- Delivering other drugs or substances directly to the cancer cells.
It should be noted that many of the recently approved immunotherapies used in treating lung cancer patients are monoclonal antibodies. See Chapter 4: Systemic Therapy for Non-Small Cell Lung Cancer
Domvanalimab is a humanized IgG1 anti-TIGIT antibody that is being evaluated in several early phase trials in combination with other antibodies (zimberelimab with or without the addition of etrumadenant). Domvanalimab is also being evaluated in combination with durvalumab in the phase III Pacific-8 trial which is currently enrolling patients.1
Vibostolimab is another anti-TIGIT antibody that is currently being evaluated in a phase III trial in combination with pembrolizumab. The initial phase 1 trial data showed ORR of 3% in both monotherapy and vibostolimab plus pembrolizumab combination therapy arms, with median OS of 11 months and 13 months, respectively.2
Etigilimab and ociperlimab are two additional anti-TIGIT antibodies that are being evaluated in early stage clinical trials for NSCLC in combination with anti-PD-1 antibodies and/or chemotherapy.3
Antibody-Drug Conjugates (ADCs)
Antibody-drug conjugates are formed when an antibody is fused with a drug via a linking structure. The antibody portion serves to identify and target cancer cells, allowing for delivery of the cytotoxic “payload” directly to the tumor. This technique has the potential to improve therapeutic response while minimizing the risk for side effects. Resistance to these types of therapies can develop when either the cell decreases expression of the antibody target, when there is decreased internalization of the drug payload, or when there is increased export of the therapeutic compound out of the cell before it can have an anti-tumor effect.
Ado-trastuzumab emtansine (T-DM1) is an ADC directed against HER 2. Although it appeared to be quite effective in breast cancer, the impact in lung cancer has been less significant. In a trial with 49 patients, there were only 4 partial responses, and these were seen only in those patients 7 with the highest level of HER2 overexpression. Median progression free survival was 2.7 months. TDM1 was well tolerated with fatigue being the only grade 3 adverse event reported in more than one patient. Also noted were risks of infusion related reactions and thrombocytopenia.4
Datopotamab deruxtecan (Dato-DXd) is an ADC directed against Trop-2. It is an IgG1 monoclonal antibody that is linked to a topoisomerase I inhibitor payload (deruxtecan). It is currently being evaluated in the TROPION-Lung02 study in combination with pembrolizumab. In previously treated patients, the RR was 39% with a DCRC of 86%. For the first-line cohort, the ORR was 69% and the DCR was 100%.5 Side effects included stomatitis (irritation of the oral mucosa) nausea/vomiting, fatigue, and alopecia. Dato-DXd continues to be studied in combination with both chemotherapy and immunotherapies.
Trastuzumab deruxtecan (DS-8201), is a HER2 antibody–drug conjugate made up of an anti-HER2 monoclonal antibody that is linked to a topoisomerase I inhibitor. In the Phase 2 DESTINY-Lung01 study evaluating patients with HER-2 mutation, ORR was 72%, with median PFS of 8.2 months. The most common grade 3 or higher side effect was neutropenia in 19% of patients, with lung toxicity noted in 26% of patients including two fatal events of lung toxicity.6 Further testing of this compound is ongoing in the DESTINY-Lung02 trial; ClinicalTrials.gov number, NCT04644237.7
Patritumab deruxtecan (U3-1402) is an ADC directed against HER3. Its toxic therapy is DC 8951, which is a topoisomerase 1 inhibitor. It is currently being evaluated in a phase 1 clinical trial for patients whose metastatic NSCLC has an EGFR mutation and whose disease has progressed on an oral EGFR directed targeted therapy. 6It has shown the ability to overcome resistance mechanisms such as C797S, T790M, HER2, and CDK4 amplifications.8 Initial trial results were promising, with an ORR of 39% and median PFS of 8.2 months.9 The most common side effects were hematologic, including decreased neutrophil count, anemia, and decreased platelet count. It was well tolerated with only 9% of patients discontinuing treatment due to adverse effects. Phase ½ trials NCT03260491 and NCT02980341 are ongoing.10
Sacituzumab govitecan (IMMU-132) is an ADC targeting Trop-2 that is combined with a toxic payload of SN-38, the active metabolite of irinotecan and a topoisomerase 1 inhibitor. Fifty four heavily pretreated NSCLC patients were evaluated in a phase 1 expansion cohort with a demonstrated response rate of 17%, with median PFS of 5.2 months and median OS of 9.5 months. However, 67% of patients were seen to have a reduction of tumor size from baseline. Side effects included neutropenia, GI upset (diarrhea, nausea) and fatigue.11 Additional trials are underway to evaluate Sacituzumab govitecan in combination with pembrolizumab12 as well as in the second line setting for salvage therapy after progression on combination chemo and immunotherapies.13
Telisotuzumab Vedotin (ABBV-399) is a c-Met–targeted antibody-drug conjugate. Results of the Phase 1b study of telisotuzumab vedotin in combination with erlotinib in patients with lung cancer harboring an EGFR mutation and c-Met amplification showed a response rate of up to 35%. Side effects included rash, diarrhea, nausea, vomiting, fatigue, neuropathy, and loss of appetite. An increased risk of blood clots was seen as well.14
In the Phase 1/2 LUMINOSITY trial (NCCT03539536) the ORR in pts with previously treated c-Met overexpressing non-squamous EGFR WT NSCLC was 36.5%, with high c-Met group showing a response rate of 52.2%. An arm for patients with high c-Met expressing tumors is being investigated in a Stage 2 expansion cohort.15
Additional ADCs currently under investigation include Tusamitamab ravtansine (SAR408701), zanidatamab zovodotin (ZW49), DS-7300, and enopotamab vedotin (EnaV).16
Targeted Therapy - Small Molecules
Small molecule drugs are a large class of medications that work by entering the cell and blocking the sequence of reactions that cause cellular proliferation. By blocking this sequence of reactions in cancer cells, the small molecule drugs kill the cancer cells and slow or stop tumor growth. In normal cells, tyrosine kinase enzymes activate a phosphorylation cascade that regulates signals sent to the cell nucleus and governs the timing of cellular proliferation, differentiation, and programmed cell death (apoptosis). In malignant cells, this communication cascade may be switched on permanently, resulting in unregulated cellular proliferation and tumor growth. Tyrosine kinase inhibitors are small molecule drugs that interfere with this sequence of reactions, stopping cell proliferation and causing cell death. New tyrosine kinase inhibitors continue to be studied for use in lung cancer, and several are now commercially available for patients with specific, targetable mutations in tumor DNA. During treatment with small molecules the cancer cells may develop additional mutations that confer resistance to first line therapy. Identification of second and third-line therapies that continue to exploit the underlying driver mutation but also block resistance mutations has become increasingly important. EGFR and ALK are two well established therapeutic targets for small molecule inhibitors. However, multiple newer targets continue to be identified, offering patients a chance at treating their disease while maintaining better quality of life with fewer side effects than they might have with chemotherapy.
EGFR inhibitors
Multiple EGFR targeting drug therapies have been developed for lung cancer over the past 10 years. The challenge now is to identify which therapies will allow patients to remain on targeted therapy for longer, and maximize clinical benefit either by blocking resistance mechanisms from the outset, or by adding in additional therapies to address resistance mechanisms as they develop over time. Multiple early stage trials are currently underway to identify promising drugs that may be used to combat the resistance mechanisms that arise following first line targeted therapies.
BLU-945 is a fourth generation EGFR inhibitor that has activity against both “double mutants” with EGFRm and T790m, as well as “triple mutants” with EGFRm, T790m and C797s.17 Both T790m and C797s mutations have been well documented as resistance mechanisms to EGFR TKI drugs. Fourth generation compounds such as BLU-945 seek to block not only the original EGFR mutation, but also the more common resistance mechanisms which may develop, thereby prolonging a patient’s response and extending the time to progression on first line therapy. A phase 1/2 trial (NCT04862780) is currently ongoing to further evaluate the safety and efficacy of this compound.18
Nazartinib (EGF816) is a third generation EGFR inhibitor with activity against exon 19 deletion, L858R and T790m mutations. It is currently being evaluated in Phase III trials in the first-line setting. Common side effects included rash, diarrhea, itching, mouth sores, and fatigue. Response rate in the Phase 2 trial was 64%, The 6-month duration of response rate was 91%, and the median duration of response was not estimable. Nazartinib also demonstrated good intracranial efficacy, with 53% of patients with baseline brain metastasis experiencing resolution of their intracranial disease. Currently this drug is available only through clinical trials.19
Because of the higher prevalence of EGFR mutations in Asian patients, there are several trials being conducted exclusively in Asia to evaluate the safety and tolerability of novel EGFR directed compounds. Unfortunately, all patients on EGFR targeted therapies will eventually progress, and much effort is being focused on how to extend time to progression on first line therapy, as well as how best to identify and treat resistance mechanisms that can arise. In the front line setting osimertinib is being combined with earlier generation EGFR TKIs (NCT03122717 ), with chemotherapy such as carboplatin and pemetrexed, as well as with VEG-F inhibitors including bevacizumab and ramucirumab. It is critical to obtain additional molecular profiling after progression of first line therapy to try to identify the nature of the acquired resistance mechanism as that can guide the selection of subsequent therapy. For example, if a MET alteration is identified then a trial combining osimertinib with a MET inhibitor such as tepotinib20 or savolitinib21-23 could be considered. Alternatively, if a C797S mutation is identified, then changing to a first generation ERGFR TKI such as erlotinib or gefitinib may be appropriate. For those patients who developed a “triple mutation” with EGFR m+/T790m/C797S mutations, promising results were also seen when combining brigatinib (a dual EGFR/ALK TKI) with either cetuximab, or a combination of bevacizumab + osimertinib.24-27
Additional cell signaling pathways and drug combinations being evaluated include blockade of MEK (osimertinib + selumetinib), and Aurora Kinase A pathways (osimeritnib + alisertib) or inhibiting mTOR (Osimertinib + sapanisertib) (Results of a phase 1b study of osimertinib plus sapanisertib or alisertib for osimertinib-resistant, EGFR-mutant non–small cell lung cancer (NSCLC).28
The ORCHARD trial was designed to answer the urgent question of how best to treat EGFRm+ patients after progression of disease on first line osimertinib. Patients are matched with second line treatment based on repeat molecular profiling performed after progression on first line osimertinib. Several targeted therapies are being investigated in combination with osimertinib (savolitinib, gefitinib, necitumumab, alectinib, selpercatinib, selumetinib, and datopotamab deruxtecan). Comparator arms include osimertinib + chemo, chemo + durvalumab or an observational standard of care arm to evaluate based on current best practices.29
Data from some of the arms of the ORCHARD trial are available, including the osimertinib + necitumumab arm and the durvalumab + chemotherapy arm, both of which met futility/stop criteria and discontinued enrollment.30-31
ALK inhibitors
Since its identification as a therapeutic target in NCSLC, multiple drugs have been FDA approved for use in patients with an ALK rearrangement (aka mutation). Similarly to what is observed in the EGFR mutant patient population, despite initial excellent response rates to TKI therapies, essentially all patients will eventually develop resistance and progress. Much effort is being dedicated into understanding the ideal sequencing of ALK inhibitors to obtain the best clinical response for patients, as certain resistance mechanisms are more likely to develop after some of the drugs than others. The G1202R mutation is identified as a resistance mechanism in up to 43% of patients who progress on ALK targeted therapies.32 It can only sometimes be overcome by treatment with the third generation drug lorlatinib. In addition to the single agent therapies being evaluated for use in ALK mutation positive patients described below, multiple trials are looking at combinations of ALK TKIs with other drugs, including MEK, VEGF and mTOR inhibitors.33
TPX-0131 is a novel ALK inhibitor that demonstrated potent activity against many known ALK resistance mutations in pre-clinical studies, most excitingly it was active against G1202R as well as all the known compound or “double” resistance mutations which have been challenging to target previously. A phase 1/2 trial (FORGE-1) evaluating TPX-0131 is currently enrolling patients.34
Ensartinib (X-396) is a potent ALK inhibitor with anti-cancer activity against both treatment naïve tumors and those that developed resistance to first-line therapy with crizotinib. In the Phase 1 trial over 80% of patients responded to therapy, with observed activity in the brain.35 Response lasted for a median duration of more than 20 weeks with some responses lasting for over 50 weeks. Common side effects included rash, fatigue, nausea, vomiting and swelling. Responses were also observed in the central nervous system, with an intracranial response rate of 64%. Phase 3 data from the eXalt3 trial comparing TKI treatment naïve patients randomized to crizotinib vs ensartinib showed improved PFS in the ensartinib arm and time to treatment failure at 12 months was 4.2% for ensartinib vs 23.9% for crizotinib.36 Ensartinib also conferred improved disease control in the brain, with an intracranial ORR of 64% on the ensartinib arm compared to 21% with crizotinib. Safety data was consistent with earlier trials, with ensartinib being well tolerated. One unique AE was a sunburn like rash that was typically mild. However, it is unclear how this agent would fit into the treatment paradigm, as it does not appear to have unique properties when compared to current ALK-inhibitors that are already FDA approved.
ROS-1 Inhibitors
Because the ROS1 signaling receptor target is conformationally quite similar to that of ALK and TRK, lorlatinib (FDA approved for use with ALK rearrangements) is also being evaluated for efficacy against ROS1 in Phase 2 trials, with promising results.37 Although not FDA approved, lorlatinib is recommended by NCCN guidelines for second-line therapy in patients with ROS1 rearrangement after they progress.38
Taletrectinib (AB-106 / DS-6051b) is a new selective ROS1/NTRK inhibitor that showed promising Phase 1 data and is now in Phase 2 trials across North America, Europe and Asia. The ongoing TRUST study is evaluating both TKI naïve and crizotinib resistant patients. Preliminary results showed an ORR of 90% in the treatment naïve group and 47.6% in the crizotinib resistant group. The disease control rate was 95% in the naïve group and 70% in the resistant cohort. The compound is exciting to researchers as it has been shown to have activity against the secondary resistance mutation G2032R, which commonly develops after first-line ROS directed targeted therapies such as crizotinib and confers resistance to the next-generation inhibitors lorlatinib and entrectinib. Common toxicities included nausea, vomiting, diarrhea, and lab abnormalities (cytopenias and LFT elevation).39
Foritinib (SAF-189s) is a potent oral TKI with activity against both ALK and ROS. It has demonstrated activity against multiple resistance mechanisms, including the G2032R resistance mutation that often develops after initial ROS-1 directed TKI therapy. It is currently being studied in a phase I/II trial enrolling both TKI naïve as well as pre-treated patients.
Phase 2 data showed an ORR for ROS1 TKI-naive patients of > 80%, and a disease control rate of >94%, while the ORR for crizotinib pretreated patients was 40% and DCR 72%. Foritinib also showed good control of disease in patients with metastases to the brain at baseline, with an ORR of 86%. The most common adverse effects included transaminitis, nausea and diarrhea.40-41
Ceritinib has been shown to have activity in the front-line setting for lung cancer patients whose tumors have a ROS-1 rearrangement. Unlike for those patients with ALK rearrangement, ceritinib does not seem to have second-line activity after progression on crizotinib for ROS1-rearranged tumors. A Phase 2 trial with 32 patients showed a 62% response rate and 81% disease control rate. Activity in the brain was demonstrated, with a 63% disease control rate, although the sample size was small at only 5 patients with brain metastases.42 Common side effects include diarrhea, nausea, vomiting, loss of appetite, and lab value changes including increased liver function enzymes and low phosphate levels.43 Currently ceritinib is recommended in the NCCN guidelines for first-line use in ROS1rearranged NSCLC, but it does not yet have FDA approval in this indication.44
Cabozantinib is a multi-kinase inhibitor with activity against several cell signaling targets including ROS-1 rearrangement. It is being evaluated in lung cancer following progression on crizotinib and ceritinib. In particular, it has shown efficacy against the G2032R and L2026M resistance mutations found in ROS-1 rearranged tumors.45-46 Safety and efficacy of cabozantinib continues to be evaluated in lung cancer, but common side effects when used in other types of cancer included nausea, diarrhea, fatigue, mouth sores, and hand-foot syndrome (redness, pain, tingling and numbness to hands and feet).47
Repotrectinib has demonstrated safety and activity in clinical trials (TRIDENT-1) for patients with advanced ROS-1 fusion NSCLC, with response rates of 91% in the TKI-naïve patient population and 40% in patients who had received previous ROS-1 targeted therapy.48 Repotrectinib also showed a potential to overcome TKI resistance mutations after treatment with crizotinib. In particular, the response rate in patients who developed the G2032R resistance mutation after front line TKI therapy showed a response rate of 58%. Repotrectinib also demonstrated efficacy in the brain, with an intracranial response rate of nearly 90% in TKI naïve patients, and nearly 50% in patients who had received prior TKI therapy.49 It is generally well tolerated, with dizziness, fatigue, constipation, taste changes, dyspnea and hypoxia seen in some patients. It remains available through clinical trials only, and was granted fast track status by the FDA in August 2020.50
BRAF Inhibitors
BRAF mutations are present in 4-5% of NSCLC patients. They are divided into three main classes – Class I are the V600 mutations, for which dabrafenib and trametinib are approved. Class II and III do not yet have FDA approved targeted therapies. Several combination therapies have appeared promising in the pre-clinical setting and are now being evaluated in human clinical trials. Some of these include lifirafenib (a RAF dimer inhibitor) plus mirdametinib (a MEK inhibitor) (NCT03905148) which enrolled multiple tumor types in the phase 1 cohort and showed a 23% ORR. Dose expansion is planned for 2023.51 Additional combination therapies are being evaluated in BRAF mutation positive melanoma patients, for example LXH254 with either LTT462 or trametinib or ribociclib in melanoma patients (NCT04417621). Encorafenib (Braftovi) is a BRAF inhibitor currently being studied in clinical trial in combination with binimetinib (a MEK inhibitor) in patients with advanced NSCLC whose tumors have a BRAF V600E mutation. Trial enrollment began in June 2019, no results in lung cancer patients have been published to date. The combination was approved for melanoma patients with this mutation in 2018. Side effects have been shown to include fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia. This combination may be less likely to cause fevers than other BRAF targeted therapies.52
MEK inhibitor
Selumetinib is a small molecule drug that is currently approved for pediatric patients 2 years of age and older who have neurofibromatosis type 1 (NF1) and symptomatic, inoperable plexiform neurofibromas (PN). It inhibits the mitogen-activated protein kinases MEK-1 and MEK-2. It stops cellular proliferation and induces apoptosis in some cell lines.53 Common side effects include rash, diarrhea, nausea, vomiting, hypertension, visual disturbance, and decreased liver function. It has been evaluated in combination with chemotherapy for KRAS mutant lung cancer, and unfortunately was not found to improve progression free survival when combined with docetaxel in the second-line setting. The TATTON trial showed that when combined with Tagrisso for EGFR-mutant patients Selumetinib does appear to have benefit, with 34-42% of patients having partial response, and disease control rate up to 81%.54 Selumetinib continues to be evaluated in combination with immunotherapy in ongoing clinical trials.55
Binimetinib (MEK162) is being evaluated in combination with other therapeutic agents for lung cancer, including standard of care chemotherapy agents, immunotherapy, as well as other targeted therapies. Common side effects include diarrhea, fatigue, elevated pancreatic enzymes, and rash.56 It continues to be evaluated in Phase 2 clinical trial for BRAF mutant NSCLC in combination with encorafenib, as well as for ALK-rearranged NSCLC in combination with brigatinib. It remains available through clinical trials only.
NTRK Inhibitors
NTRK 1/2/3 are oncogenic driver mutations that are present in many types of solid tumors. They are identified in approximately 1% of NSCLC patients.
Selitrectinib (LOXO-195) is a selective TRK inhibitor evaluated in an ongoing phase 1 clinical trial. Early results from 29 patients showed an overall response rate of 34%, with 45% of patients harboring an acquired NTRK mutation that developed during prior TRK directed therapy having a response to LOXO-195. Common side effects included dizziness, ataxia, nausea, fatigue, lab abnormalities and abdominal pain. The estimated trial completion date is July 2024.57
Repotrectinib (TPX-005) is a TRK inhibitor that was granted fast-track status by the FDA for NTRK + cancers after prior first line TRK inhibitor. It continues to be evaluated in the TRIDENT-1 clinical trial for patients with either NTRK 1-3, ALK or ROS1 gene rearrangements. In the NTRK mutated cohort 6 patients were evaluable, with an overall response rate of 50%. It is generally well tolerated, with dizziness, fatigue, constipation, taste changes, dyspnea and hypoxia seen in some patients.50
Additional Small Molecule Inhibitors
Multiple other targets continue to be discovered for NSCLC as science advances and the ability to test patients for multiple gene targets becomes a reality. These additional gene targets include HER2, MET amplification, MET exon 14 mutation, KRAS, RET, PIK3CA, AXL and MAP2K1 (also known as MEK1). Numerous drugs are currently being investigated that show activity against one or more of these targets. Interestingly, many of these drugs have more than one intra-cellular target and are being evaluated for application in different types of cancer as well as potentially being useful for multiple different tumor mutations. As the science of tumor genetic sequencing progresses more drug-gene targets will be established to truly personalize treatment to the genetic fingerprint of an individual patient’s cancer, with continued improvement in treatment options and therapeutic outcomes for lung cancer patients.
MET
Savolitinib is a MET inhibitor currently being evaluated in combination with osimertinib for use in patients who progress on first-line EGFR TKI therapy and demonstrate a MET exon 14 deletion as a resistance mechanism. In the TATTON trial the safety and efficacy of the combination of savolitinib with osimertinib was established. Results showed a response rate of 28% – 52%, depending on what treatment the patient had received in the front-line setting.-21-22 Side effects were somewhat more severe than observed with single agent dosing, with the most frequent side effects being nausea, diarrhea, fatigue, fevers, decreased appetite, and decreased blood cell counts (white blood cells and platelets). The combination of savolitinib with osimertinib continues to be evaluated in the Phase 2 SAVANNAH trial which began enrollment in early January 2019.23
RET
TPX-0046 is a next-generation RET inhibitor. Pre-clinical data demonstrated that this compound can overcome some of the more common resistance mechanisms identified following treatment meth currently available RET inhibitors. A phase I/II clinical trial (NCT04161391) is currently enrolling, but data from human studies are not yet available.58 Several additional compounds are in early stages of development, including TAS0953/HM06 (NCT04683250) and BOS172738 (NCT03780517). No safety or efficacy data have been published at this time.59-60
KRAS
KRAS mutations are among the most commonly found oncogenic drivers of tumorigenesis in lung cancer, however KRAS has historically been considered an “undruggable” mutation due to a lack of a traditional small-molecule binding pocket on the protein. The most common types of KRAS mutations found in lung cancer are KRAS G12C, G12D and G12V. Sotorasib was approved in the spring of 2021 for second line treatment in patients with KRAS G12C mutations. However, this is only one of the multiple oncogenic KRAS mutations that have been identified.
Adagrasib (MRTX849) is an inhibitor of KRASG12C that irreversibly and selectively binds to KRASG12C, preventing downstream cell signaling. The KRYSTAL-1 trial evaluated 51 patients with previously treated KRAS G12C mutated NSCLC, and 45% had at least a partial response to adagrasib therapy. In those patients with a concomitant STK11 mutation, the response rate was 64%.61 Side effects included GI upset (nausea, vomiting, diarrhea) fatigue and lab abnormalities.42
Adagrasib was approved by the FDA in December 2022 for second line treatment of NSCLC patients with KRAS G12C mutations.62 It continues to be evaluated in the first line setting, as well as for patients with other types of KRAS mutations.63
Multiple additional trials are ongoing to better understand the efficacy of both adagrasib and sotorasib in the first line setting, as well as in combination with immunotherapy and other targeted therapies.
EGFR / HER2 Exon 20 insertion
EGFR exon 20 insertion is a unique group of mutations. Unlike the majority of EGFR mutations which are “sensitizing” mutations, exon 20 insertion mutations tend to confer resistance to the typical first-generation EGFR TKIs. In addition to differences from the “sensitizing” EGFR mutations, these exon 20 mutations are also unique from each other, as the specific mutation location will impact response to targeted therapies.64 Thus, addressing this heterogeneous group of oncogenic targets has required development of its own set of therapies.
Poziotinib is an oral tyrosine kinase inhibitor that is being evaluated in the Phase II ZENITH20 trial for lung cancer patients with EGFR or HER2 Exon 20 insertion mutations. Poziotinib irreversibly blocks signaling through the HER family of receptors, including HER1 (ErbB1/EGFR), HER2 (ErbB2), and HER4 (ErbB4). Response rates were encouraging, with tumor reduction seen in up to 48% of patients treated. The exact location of the EGFR insertion mutation was shown to affect response rates, which varied from 48% to 0% based on the specific location of the mutation.64 Unfortunately, side effects have been problematic, with over 50% of patients experiencing moderate to severe symptoms, including rash, diarrhea, paronychia, mucositis, and fatigue. These side effects often require dose reduction or treatment discontinuation. Splitting the dose into twice daily dosages appears to decrease the risk for severe toxicity by up to 52% while maintaining response rates of approximately 30%. The ZENITH20 trial continues enrolling patients to better understand the efficacy of the twice daily dosing schedule.65
CLN-081 (TAS6417) is an oral TKI targeting EGFR exon20 insertion mutations. It is selective for the mutant form of EGFR, which may limit side effects. Early stage trials have shown it has anti-tumor effect, with an acceptable side effect profile. Common side effects included rash, diarrhea, paronychia, stomatitis, and nausea.66
Furmonertinib is a third-generation EGFR targeting TKI. A retrospective study in China evaluated all patients with exon 20 insertion mutations treated with furmonertinib across a hospital network and identified 53 patients. Response rate was 37.7%, and toxicities were mild, mainly rash and diarrhea. Two dose levels were evaluated with no statistical differences in either response rates or toxicity between the groups.67
Sunvozertinib (DZD9008) is an oral, irreversible, and selective EGFR TKI that has demonstrated activity against EGFR exon 20 insertion mutations. Early phase studies in China suggested efficacy both in the brain as well as after prior targeted therapy such as amivantimab.68 The ORR was 50%, and it demonstrated a tolerable safety profile with mainly mild to moderate rash and diarrhea.68 It was granted FDA Breakthrough Therapy Designation in January 2022. It is currently under investigation in the US through clinical trial NCT03974022.69
Pyrotinib is another TKI with irreversible binding and pan-HER activity (i.e. it has activity against HER1 (EGFR), HER2 and HER4. It has been evaluated in several Phase 2 trials, in one case after patient had received at least one prior line of chemotherapy, with almost 60% having had at least 2 prior lines of chemotherapy.70 Overall response rate was 30% with a median PFS of 6.9 months.71 Side effects were reported to be diarrhea as well as lab abnormalities.
Tarloxotinib is a pan-ErbB kinase inhibitor that was shown in pre-clinical studies to have activity against EGFR exon 20 and HER2 mutant NSCLC. The RAIN-701 study showed positive results in their first presentation of initial data analysis in September of 2020. At the time 23 patients (of a planned 60) had been enrolled onto the trial with evaluable results. Two patients had a partial response, with a demonstrated disease control rate of 60% (at least stable disease). Side effects seen included changes to heart rhythm, rash, diarrhea, nausea and lab abnormalities.72
AXL
AXL is a cell membrane receptor that has been identified new drug target in lung cancer. High AXL expression is thought to suppress the body’s immune response to cancer cells, and promotes resistance to anti-PDL1 immunotherapies.73 Further, high AXL expression is thought to confer a poor prognosis in most cancers. Currently there are no FDA approved therapies targeting AXL.
Bemcentinib (BGB324) is a novel AXL inhibitor which seemed to enhance anti-PD1 therapy in pre-clinical studies. A phase 2 trial involving 38 patients have been dosed with the combination of bemcentinib and pembrolizumab, with 29 evaluable for response to treatment. 28% of patients showed a partial response, and 40% of the AXL-positive patient responded to therapy.73 The FDA has granted bemcentinib fast track status, and clinical trials are ongoing.74
Immunotherapy
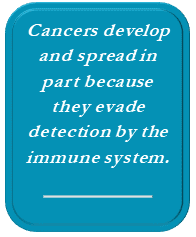 Cancers develop and spread in part because they evade detection by the immune system. The goal of immunotherapy is to make cancer cells recognized as abnormal or “non-self” by the immune system, enabling natural immune defense mechanisms to eliminate the cancer. With immunotherapy, side effects are typically mild because the drugs affect only certain types of cells, and they use the body’s own defenses (not cytotoxic drugs) to kill cancer cells. However, in some cases the immune system may become over-engaged, creating auto-immune inflammatory side effects. These side effects can be severe and may require treatment with immune suppressant medications.
Cancers develop and spread in part because they evade detection by the immune system. The goal of immunotherapy is to make cancer cells recognized as abnormal or “non-self” by the immune system, enabling natural immune defense mechanisms to eliminate the cancer. With immunotherapy, side effects are typically mild because the drugs affect only certain types of cells, and they use the body’s own defenses (not cytotoxic drugs) to kill cancer cells. However, in some cases the immune system may become over-engaged, creating auto-immune inflammatory side effects. These side effects can be severe and may require treatment with immune suppressant medications.
Several antibodies have been developed that target immune checkpoints, which play a role in cell signaling and driving cancer growth. Some of the most promising developments in treating lung cancer have been seen with drugs that target the Programmed Death 1 (PD-1) receptor pathway, including Opdivo (nivolumab), Keytruda (pembrolizumab), Tecentriq (atezolizumab), and Imfinzi (durvalumab). Currently all four of these have been approved by the FDA for at least one indication in lung cancer treatment.
Ipilimumab and tremelimumab are monoclonal antibodies that inhibit the cytotoxic T-lymphocyte- associated protein 4 (CTLA-4) immune checkpoint pathway. They are now approved for use in lung cancer in combination with anti-PD1 targeting agents as well as chemotherapy. They have a similar side effect profile to the Anti-PD1 antibodies, including rash, diarrhea/colitis, hepatitis, iritis/uveitis, hormonal changes, and pneumonitis. However, side effects tend to be more common with Anti-CTLA-4 drugs compared to Anti PD-1 and Anti-PD-L1 compounds.75-76
APX005M is a CD40 Agonistic Antibody being studied in combination with nivolumab and cabiralizumab. This drug was designed to stimulate the immune system and enhance the anti-cancer immune response. There is limited data for NSCLC, however a Phase 1 trial reported on four patients of whom one had a partial response, two had stable disease and the fourth progressed.77 Side effects include fatigue, malaise, nausea, fevers, chills and flu like symptoms. The Phase 2 trial continues to enroll patients, with results forthcoming.
NC318 is a novel monoclonal antibody targeting Siglec-15. It was designed to function in the tumor microenvironment and enhance t-cell function, thereby restoring the ability of the immune system to recognize and fight off cancer cells. Currently only pre-clinical data have been published and suggest a tolerable safety profile with less risk of serious immune-related toxicity than the anti-PD-1 and anti-CTLA4 targeted antibodies have previously demonstrated.78 The initial Phase1/2 clinical trial is currently enrolling patients and has an expected completion date 2021.
To try to both improve first line response rates to immunotherapy as well as to overcome resistance to first line immunotherapies, multiple trials are being conducted evaluating novel combinations of immunotherapy with targeted therapies. Some of these include PARP inhibitors, VEGF inhibitors, novel anti-CTLA-4 antibodies, anti-CD137, anti-LAG3 and anti-TIM3 antibodies, as well as ICOS agonists and antagonists.
While the compounds noted above (and the majority of lung cancer clinical trials) continue to be designed to help patients diagnosed with stage IV disease, multiple immunotherapies and targeted therapies are also being evaluated for use in patients diagnosed with earlier stage lung cancers because of their potential for considerable clinical benefit, and several have now been approved by the FDA.
Another type of cancer treatment that has received a lot of attention recently is CAR-T therapy. It is currently approved for several types of blood cancers, but its utility in solid tumors, and in lung cancer remains unclear. Part of the challenge in lung cancer is identifying a target unique to the cancer cells that are not present elsewhere in the body. Too much overlap between the presence of the target in the tumor and healthy tissues will result in the patient suffering severe side effects due to off target impacts of the treatment. Rather than CAR-T cells, a perhaps more promising avenue in solid tumor is to develop specific tumor infiltrating lymphocytes (TILs) that are unique to a particular patient and will recognize and eliminate their specific cancer cells. Trials are currently enrolling patients where researchers collect a tumor specimen from a patient and then isolate the active TILs, increase their number exponentially in a lab, and then infuse them back into the patient. The TIL therapy regimen is quite intense, almost like a mini bone marrow transplant. First the patient undergoes a biopsy to collect tumor tissue. If adequate tissue is obtained, then the patient is admitted to the hospital for several weeks of chemotherapy. During this time the TILs are being isolated and grown in the lab. If the patient is clinically stable after the chemotherapy, they will then receive the infusion of TILs along with other immune stimulatory agents, after which they a monitored closely for potentially severe side effects.
Tumor Mutational Burden
Immune Checkpoint Inhibitors have become a mainstay of lung cancer treatment. In patients without targetable mutations immunotherapy continues to show improved efficacy either as monotherapy or in combination with chemotherapy in comparison with chemotherapy alone in the first- and second-line setting. Unfortunately, only about 20% of patients demonstrate durable long-term responses to these drugs, while a significant proportion of patients experience disease progression within the first months of treatment. PD-L1 expression level is currently the only biomarker approved by the FDA, yet it is not perfect, and seems to correlate with treatment response in some but not all patients. One cannot ignore the high cost of these immunotherapy medications (as much as $150,000/year), and from a purely economic perspective a better marker to identify response is needed to avoid wasteful spending of healthcare dollars. For these reasons, a new set of biomarkers must be developed that will better guide clinicians and help identify those patients who will benefit from immune checkpoint inhibitors.
Tumor mutational burden (TMB) is one potential marker of response to immunotherapies. TMB is defined as the number of mutations per DNA megabase. It continues to be studied as a potential biomarker to predict response to immune checkpoint inhibitors in NSCLC. To date, responses from clinical trials have been mixed, with some trials showing a predictive association between high TMB and treatment response, while others not finding the same predictive relationship.79-80
Because of the potential for long-term durable responses with immunotherapies, any marker selected to guide treatment selection will have to be well validated with extensive clinical support. Limiting treatment options and potentially preventing patients from the benefit of these novel therapies needs to be considered with the utmost caution.
Corticosteroids and ICI
Recent research has brought into question whether steroid use affects clinical outcomes during treatment with immunotherapies. Some studies have demonstrated that patients who received corticosteroids prior to starting ICI therapy experienced lower overall response rates, worse progression free survival, and poorer overall survival.81
One large retrospective study looked to determine the effect of corticosteroid use specifically in the treatment of immune-related adverse effects and found that there was no significant difference in overall survival (median; 14.5 vs 30.0 months), progression-free survival (median; 7.8 vs 9.6 months), and objective response rate (46% vs 41%) in patients who required steroids (>10mg per day) vs those who did not. They concluded that steroids should not be avoided in patients with moderate to severe immune-related adverse effects due to concerns over reduced efficacy.82
Gut Microbiome and Cancer Treatment
Another avenue of research in cancer treatment is the effect of the gut microbiome on overall health, promotion of pathogenic conditions including tumor development and treatment outcomes. A recent report documented stark differences in gut flora of patients following cancer treatments compared to their healthy peers. It has long been understood that antibiotics can change the gut microbiome, and it has become clear that use of antibiotics can impact response to immune checkpoint inhibitor therapy. Several studies have demonstrated significant reduction in progression free and overall survival when antibiotics are administered in the 30 days prior to initiation of immunotherapy treatment.83-85 Methods for how best to restore gut balance and enhance the positive effects of the microbiome on health are currently under investigation.
Vaccines
Vaccines are also being used to treat lung cancer and as maintenance therapy with the goal of decreasing or preventing the risk of recurrence. Analogous to vaccines that may prevent the spread of communicable diseases, these cancer vaccines stimulate the immune system to identify and attack cancer cells without damaging normal cells.
CIMAvax-EGF is also known as the “Cuban Vaccine” and is currently available in the US through clinical trials. Multiple trials have shown that it is safe and does elicit an immune response.86 Side effects have been reported to include fevers, injection site irritation, vomiting, and headache. A phase III trial evaluating use of the vaccine after completion of platinum chemotherapy did not find overall survival benefit.87 Further studies are needed to determine whether this vaccine can benefit lung cancer patients.
TG4010 is targeted immunotherapy based on a pox virus (the Modified Vaccinia Ankara virus) that codes for the MUC1 tumor-associated antigen and interleukin-2. TG4010 has been assessed in combination with first-line chemotherapy in advanced NSCLC and has shown an improvement in progression-free survival.88 Common side effects include injection site reaction and flu-like symptoms.89 TG4010 continues to be evaluated in Phase 2 and 3 clinical trials, and is now being combined with chemotherapy as well as Opdivo (nivolumab).90
BI 1361849 (CV9202) is a vaccine that is made up of six mRNAs that code for six different NSCLC-associated antigens. Phase 1 trials have demonstrated safety and tolerability of the compound, as well as an enhanced anti-tumor effect when combined with radiation.91 Collection of survival data remains ongoing. The most common side effects were injection site reactions and mild to moderate flu like symptoms. As it is thought that this compound may increase tumor infiltrating lymphocytes, a trial combining it with an immunotherapeutic agent targeting Anti-PD-1 was performed with modest responses seen in the 26 enrolled patients, including one partial response and 46% of patients with stable disease.92 A trial is ongoing combining BI1361849 with both Anti-PD-L1 and Anti-CTLA-4 antibodies.93
Chemotherapy
Although much research is focusing on new approaches to lung cancer treatment, research also is being done to develop new drugs for chemotherapy or improve existing chemotherapy regimens. Combination therapy has long been the hallmark of cancer treatment. As promising new agents are identified, they are evaluated in clinical trials in an effort to identify novel treatment modalities that will improve quality of life and prolong survival. Multiple trials evaluating the addition of small molecules, monoclonal antibodies, as well as immunotherapy are currently underway.
Chemoprevention
Multiple studies have been conducted to identify compounds that might prevent the development of lung cancer. Unfortunately, to date none have been identified that have demonstrated a dramatic decrease in cancer rates.94 Antioxidants and anti-inflammatory drugs like COX-2 inhibitors did not ultimately show a decreased cancer incidence, but aspirin seemed to slightly decrease risk in several studies, particularly in those at highest risk for developing lung cancer.95 A better understanding of features of pre-malignant lesions continues to develop, and a personalized “cocktail” may ultimately offer the best protection against developing lung cancer in high risk individuals.96 Unfortunately no compound has been identified that has protective effects against lung cancer. Smoking cessation remains the best approach available to prevent an individual from developing lung cancer.
Lung Cancer Screening Programs
 Because 1 in 9 smokers will go on to develop lung cancer, avoiding exposure to tobacco smoke and smoking cessation remain the best defense against lung cancer.96 See Chapter 13: How to Quit Smoking Confidently and Successfully. However, novel screening algorithms are being developed for use of low-dose screening CT scans in order to identify both those individuals at highest risk for lung cancer, as well as to identify cancers in an early, asymptomatic, surgically resectable and thus more treatable stage. The best lung cancer screening programs are comprehensive, and include the services of pulmonary experts, as well as oncologists and counselors who can educate patients regarding their risk of developing cancer as well as interpret and appropriately act on any screening test results.
Because 1 in 9 smokers will go on to develop lung cancer, avoiding exposure to tobacco smoke and smoking cessation remain the best defense against lung cancer.96 See Chapter 13: How to Quit Smoking Confidently and Successfully. However, novel screening algorithms are being developed for use of low-dose screening CT scans in order to identify both those individuals at highest risk for lung cancer, as well as to identify cancers in an early, asymptomatic, surgically resectable and thus more treatable stage. The best lung cancer screening programs are comprehensive, and include the services of pulmonary experts, as well as oncologists and counselors who can educate patients regarding their risk of developing cancer as well as interpret and appropriately act on any screening test results.
Conclusion
Lung cancer is a devastating diagnosis, but research is improving both the options for treatment of this disease as well as patient outcomes. Chemotherapy has been the mainstay of treatment for most advanced lung cancers for many years. However, new targeted therapies and immunotherapies are changing the treatment landscape for lung cancer, with several new drugs demonstrating remarkable improvement in patient outcomes in terms of both progression free and overall survival. Additional novel agents used both alone and in combination with existing agents are being studied in clinical trials, with the goal of further improving patient outcomes and survival. More therapies will become available in the near future. With advances in lung cancer treatment, patients will benefit from treatments that have fewer side effects and provide long term responses to treatment, such that even if lung cancer remains incurable, it may be treated as a chronic disease and managed for many years.

Questions to Ask About Clinical Trials
References
- A Global Study to Assess the Effects of Durvalumab + Domvanalimab Following Concurrent Chemoradiation in Participants With Stage III Unresectable NSCLC https://www.clinicaltrials.gov/study/NCT05211895 [last accessed 11/30/2023]
- Rousseau 1, C. Parisi 1, F. Barlesi Anti-TIGIT therapies for solid tumors: a systematic review. ESMO Open Volume 8, Issue 2, April 2023, 101184
- Rotte A, Sahasranaman S, Budha N. Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. 2021; 9(9):1277. https://doi.org/10.3390/biomedicines9091277
- Peters et al 2019: Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2- Overexpressing Metastatic Non–Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers | https://pubmed.ncbi.nlm.nih.gov/30206164/
- Verma S, Breadner D, Raphael J. ‘Targeting’ Improved Outcomes with Antibody-Drug Conjugates in Non-Small Cell Lung Cancer—An Updated Review. Current Oncology. 2023; 30(4):4329-4350. https://doi.org/10.3390/curroncol30040330
- Bob T. Li, M.D., Ph.D., M.P.H., Egbert F. Smit, M.D., Ph.D., Yasushi Goto, Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer M.D January 20, 2022 N Engl J Med 2022; 386:241-251 DOI: 10.1056/NEJMoa2112431)
- Trastuzumab Deruxtecan in Participants With HER2-mutated Metastatic Non-small Cell Lung Cancer (NSCLC) (DESTINY-LUNG02) https://clinicaltrials.gov/study/NCT04644237
- Ying Meng, Rilan Bai, Jiuwei Cu; Precision targeted therapy for EGFR mutation-positive NSCLC: Dilemmas and coping strategies Thoracic cancer. First published: 02 April 2023 https://doi.org/10.1111/1759-7714.14858
- U Pasi Janne, Christina Baik, Helena Yu et al. 3-1402 in Metastatic or Unresectable Non-Small Cell Lung Cancer Cancer Discov (2022) 12 (1): 74–89.
- Phase I/II Study of U3-1402 in Subjects With Human Epidermal Growth Factor Receptor 3 (HER3) Positive Metastatic Breast Cancer https://clinicaltrials.gov/study/NCT02980341
- Therapy of Advanced Non–Small-Cell Lung Cancer With an SN-38-Anti-Trop-2 Drug Conjugate, Sacituzumab Govitecan | https://pubmed.ncbi.nlm.nih.gov/28548889/
- Study of Sacituzumab Govitecan Combinations in First-line Treatment of Participants With Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) (EVOKE-02) https://clinicaltrials.gov/study/NCT05186974
- Study of Sacituzumab Govitecan (SG) Versus Docetaxel in Participants With Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) (EVOKE-01) https://clinicaltrials.gov/study/NCT05089734
- Ross Camidge, Fabrice Barlesi, Jonathan Wade Goldman, et al. Results of the phase 1b study of ABBV-399 (telisotuzumab vedotin; teliso-v) in combination with erlotinib in patients with c-Met+ non-small cell lung cancer by EGFR mutation status. Journal of Clinical Oncology 2019 37:15_suppl, 3011-3011.
- Ross Camidge, Jair Bar, Hidehito Horinouchi, et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-Met–overexpressing (OE) advanced non-small cell lung cancer NSCLC https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.9016
- Verma S, Breadner D, Raphael J. ‘Targeting’ Improved Outcomes with Antibody-Drug Conjugates in Non-Small Cell Lung Cancer—An Updated Review. Current Oncology. 2023; 30(4):4329-4350. https://doi.org/10.3390/curroncol30040330
- Xiaojing Du et al. Acquired resistance to third-generation EGFR-TKIs and emerging next- generation EGFR inhibitors https://doi.org/10.1016/j.xinn.2021.100103
- (SYMPHONY) Phase 1/2 Study Targeting EGFR Resistance Mechanisms in NSCLC https://clinicaltrials.gov/study/NCT04862780
- Tan DS, Kim SW, Sequist LV, et Phase II results for single-agent nazartinib (EGF816) in adult patients (pts) with treatment-naive EGFR-mutant non-small cell lung cancer (NSCLC). ESMO Conference October 19, 2018
- A Study of Tepotinib Plus Osimertinib in Osimertinib Relapsed MET Amplified NSCLC (INSIGHT 2) https://clinicaltrials.gov/ct2/show/NCT03940703
- Lecia V. Sequist, Jong Seok Lee, Ji-Youn Han, et al. TATTON Phase Ib expansion cohort: Osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; 2019 Mar 29-Apr 3; Atlanta, GA. Philadelphia (PA): AACR; Cancer Res 2019;79(13 Suppl):Abstract nr CT033.
- Helena Yu, Myung-Ju Ahn, Sang-We Kim, et TATTON Phase Ib expansion cohort: Osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior first/second-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; 2019 Mar 29-Apr 3; Atlanta, GA. Philadelphia (PA): AACR; Cancer Res 2019;79(13 Suppl): Abstract nr CT032. Phase II study of tepotinib in NSCLC patients with METex14 mutations.
- Geoffrey R. Oxnard, Mireille Cantarini, Paul Frewer, et al. SAVANNAH: A Phase II trial of osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-driven (MET+), locally advanced or metastatic non-small cell lung cancer (NSCLC), following disease progression on osimertinib. Journal of Clinical Oncology 2019 37:15_suppl, TPS9119-TPS9119
- Wang X, Zhou L, Yin JC, et al. Lung adenocarcinoma Harboring EGFR 19del/C797S/t790m triple mutations responds to brigatinib and anti-EGFR antibody combination therapy. J Thorac Oncol. 2019;14:e85–8.
- Wang Y, Yang N, Zhang Y, et al. Effective treatment of lung adenocarcinoma harboring EGFR-activating mutation, T790M, and cis-C797S triple mutations by brigatinib and cetuximab combination therapy. J Thorac Oncol. 2020;15:1369–75.
- Zhao J, Zou M, Lv J, et al. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: a case report. Onco Targets Ther. 2018;11:5545–50.
- Li, Y., Mao, T., Wang, J. et al. Toward the next generation EGFR inhibitors: an overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer. Cell Commun Signal 21, 71 (2023). https://doi.org/10.1186/s12964-023-01082-8
- Yasir Y Elamin, Marcelo Vailati Negrao, Frank V. Fossella, et al. Journal of Clinical Oncology 2022 40:16_suppl, 9105-9105).
- Phase 2 Platform Study in Patients With Advanced Non-Small Lung Cancer Who Progressed on First-Line Osimertinib Therapy (ORCHARD) https://www.clinicaltrials.gov/study/NCT03944772
- JW Riess, JA De Langen, Z Piotrowska et al: 329P ORCHARD: Osimertinib + necitumumab in patients (pts) with advanced NSCLC whose disease progressed on first-line (1L) osimertinib – Annals of Oncology
- BC Cho, M-J Ahn, C Baik et al: 13P Durvalumab + chemotherapy in patients (pts) with advanced EGFR mutation-positive (EGFRm) NSCLC whose disease progressed on first-line (1L) osimertinib: An ORCHARD study interim analysis – Annals of Oncology
- Gainor et al, Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer | Cancer Discovery (aacrjournals.org)
- Shirish Combinations: VEGF, trametinib, SHP2, mTOR Gadgeel – IASLC 2021 Targeted therapy conference. https://ttlc2021.iaslc.org/program-february/
- A Study of TPX-0131, a Novel Oral ALK Tyrosine Kinase Inhibitor, in Patients With ALK+ Advanced or Metastatic NSCLC https://clinicaltrials.gov/study/NCT04849273
- J Horn, L et A phase I trial of X-396, a novel ALK inhibitor, in patients with advanced solid tumors. J Clin Oncol 32:5s, 2014 (suppl; abstr 8030)
- Wong et al: Ensartinib as Treatment for ALK-Rearranged NSCLC : Oncology Times https://journals.lww.com/oncology-times/fulltext/2020/10200/ensartinib_as_treatment_for_alk_rearranged_nsclc.1.aspx
- Jiyun Lee, Jong-Mu Sun, Se-Hoon Lee, et al. Efficacy and Safety of Lorlatinib in Korean Non– Small-Cell Lung Cancer Patients With ALK or ROS1 Rearrangement Whose Disease Failed to Respond to a Previous Tyrosine Kinase Inhibitor, Clinical Lung Cancer, Volume 20, Issue 3, 2019, Pages 215-221, ISSN 1525-7304
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer v2.2019. © National Comprehensive Cancer Network, Inc. 2019. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Wei Li, Nong Yang, et al The efficacy and safety of taletrectinib in patients with TKI-naïve or crizotinib-pretreated ROS1-positive non–small cell lung cancer (NSCLC). Journal of Clinical Oncology 2022 40:16_suppl, 8572-8572
- SAF-189s, a potent new-generation ROS1 inhibitor, is active against crizotinib-resistant ROS1 mutant-driven tumors | Acta Pharmacologica Sinica (nature.com) https://nature.com/articles/s41401-020-00513-3
- A Phase I/II Clinical Study of SAF-189s in Non-small Cell Lung Cancer (NSCLC) Patients – Full Text View – ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04237805
- Lim SM1, al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small- Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol. 2017 Aug 10;35(23):2613-2618. doi: 10.1200/JCO.2016.71.3701. Epub 2017 May 18.
- Vivek Subbiah, Ceritinib in ALK-rearranged non-small-cell lung N Engl J Med 2014;370:1189-1197.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer v2.2017. © National Comprehensive Cancer Network, Inc. 2017. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Curtis Chong, et. al. Identification of existing drugs that effectively target NTRK1- and ROS1- rearrangements in lung cancer. DOI: 10.1158/1078-0432.CCR-15-1601 Published 1 July 2016 Future Oncol. 2013 Aug;9(8):1083-92. doi: 10.2217/fon.13.128.
- Sun, Thomas Yang et al. Lengthy Progression-Free Survival and Intracranial Activity of Cabozantinib in Patients with Crizotinib and Ceritinib-Resistant ROS1-Positive Non–Small Cell Lung Cancer, Journal of Thoracic Oncology, Volume 14, Issue 2, e21 – e24
- Viola D1, Cappagli V, Elisei R., Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid Proc Natl Acad Sci U S A. 2016 Mar 15; 113(11): E1419–E1420. Published online 2016 Feb 25. doi: 10.1073/pnas.1522052113.
- Turning Point Therapeutics Reports Early Interim Data From Registrational Phase 2 Trident-1 Study of Repotrectinib, Provides Regulatory Update – Turning Point Therapeutics, Inc. https://www.clinicaltrials.gov/study/NCT03093116
- Goodwin, Peter M. Next-Generation ROS1 Inhibitor Brings High Response Rates in Lung Cancer. Oncology Times 45(1):p 29, January 5, 2023. | DOI: 10.1097/01.COT.0000912084.37558.45
- FDA Grants Fast Track to Repotrectinib in NTRK-Positive Advanced Solid Tumors https://clinicaltrials.gov/ct2/show/NCT03093116
- Solomon, B, Gao B, Subbiah V, et al. Safety, pharmacokinetics, and antitumor activity findings from a phase 1b, open-label, dose-escalation and expansion study investigating RAF dimer inhibitor lifirafenib in combination with MEK inhibitor mirdametinib in patients with advanced or refractory solid tumors. Presented at: 2023 AACR Annual Meeting; April 14-19, 2023; Orlando, FL. Abstract CT033
- William H. Sharfman, MD, Encorafenib and Binimetinib: A New Benchmark in Metastatic Melanoma Therapy? https://ascopost.com/issues/december-10-2018/encorafenib-and- binimetinib/ [Last accessed 10/31/19]
- Garon EB, Finn RS, Hosmer W, et al. Identification of common predictive markers of in vitro response to the Mek inhibitor selumetinib (AZD6244; ARRY-142886) in human breast cancer and non-small cell lung cancer cell lines. Mol Cancer Ther. 2010;9(7):1985-94.
- Suresh S. Ramalingam, MD, MET/MEK inhibitor duo shows activity in resistant NSCLC https://www.mdedge.com/hematology-oncology/article/198628/lung-cancer/met/mek-inhibitor- duo-shows-activity-resistant-nsclc [Last accessed 10/31/19]
- Janne, P et SELECT-4: Phase I dose escalation trial of selumetinib (AZD6244, ARRY-142886) in combination with durvalumab (MEDI4736) in patients with advanced solid tumors. J Clin Oncol 34, 2016 (suppl; abstr TPS2607).
- Heigener, David et Targeting of MEK in Lung Cancer Therapeutics. Lancet Respiratory Medicine, The, 2015-04-01, Volume 3, Issue 4, Pages 319-327.
- Abstract CT127: Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi) Cancer Research (aacrjournals.org)
- The next-generation RET inhibitor TPX-0046 is active in drug-resistant and naïve RET-driven cancer models. Journal of Clinical Oncology (ascopubs.org) https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.3616
- Study of RET Inhibitor TAS0953/HM06 in Patients With Advanced Solid Tumors With RET Gene Abnormalities – https://clinicaltrials.gov/study//NCT04683250
- Safety, Efficacy, and Tolerability of BOS172738 in Patients With Advanced Rearranged During Transfection (RET) Gene-Altered Tumors https://clinicaltrials.gov/study/NCT03780517
- 99O_PR KRYSTAL-1: Activity and preliminary pharmacodynamic (PD) analysis of adagrasib (MRTX849) in patients (Pts) with advanced non–small cell lung cancer (NSCLC) harboring KRASG12C mutation – Journal of Thoracic Oncology (jto.org) https://jto.org/article/S1556- 0864%2821%2901941-9/fulltext
- FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-adagrasib-kras-g12c-mutated-nsclc
- KRYSTAL1 and 2: A phase I/II trial of adagrasib (MRTX849) in combination with TNO155 in patients with advanced solid tumors with KRAS G12C mutation. https://clinicaltrials.gov/ct2/show/NCT03785249
- Low JL, Lim SM, Lee JB, et al. Advances in the management of non-small-cell lung cancer harbouring EGFR exon 20 insertion mutations. Therapeutic Advances in Medical Oncology. 2023;15. doi:10.1177/17588359221146131
- Le X, Cornelissen R, Garassino M, Clarke JM, et al. MA. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial. J Clin Oncol. 2022 Mar 1;40(7):710-718. doi: 10.1200/JCO.21.01323. Epub 2021 Nov 29. PMID: 34843401; PMCID: PMC8887939.
- Zofia Piotrowska, Helena Alexandra Yu, James Chih-Hsin Yang, et al Safety and activity of CLN-081 (TAS6417) in NSCLC with EGFR Exon 20 insertion mutations (Ins20).. Journal of Clinical Oncology 2021 39:15_suppl, 9077-9077
- Sa, H., Shi, Y., Ding, C. et al. A real-world study of the efficacy and safety of furmonertinib for patients with non-small cell lung cancer with EGFR exon 20 insertion mutations. J Cancer Res Clin Oncol (2023). https://doi.org/10.1007/s00432-023-04726-x)
- Mengzhao Wang, James Chih-Hsin Yang, Paul L. Mitchell, et al. Sunvozertinib, a Selective EGFR Inhibitor for Previously Treated Non–Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations. Cancer Discov 1 July 2022; 12 (7): 1676–1689. https://doi.org/10.1158/2159-8290.CD-21-1615
- Assessing an Oral EGFR Inhibitor, DZD9008 in Patients Who Have Advanced Non-small Cell Lung Cancer With EGFR or HER2 Mutation (WU-KONG1) https://clinicaltrials.gov/study/NCT03974022
- Guanghui Gao, et al. ingle-arm, phase II study of pyrotinib in advanced non-small cell lung cancer (NSCLC) patients with HER2 exon 20 Journal of Clinical Oncology 2019 37:15_suppl, 9089- 9089
- Zhou C, Li X, Wang Q, et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma after Platinum-based Chemotherapy: A Multicenter, Open-label, Single-arm, Phase II J Clin Oncol. 2020;38(24):2753-61.
- First analysis of RAIN-701: Study of tarloxotinib in patients with non-small cell lung cancer (NSCLC) EGFR Exon 20 insertion, HER2-activating mutatioin. OncologyPRO (esmo.org)
- A phase II study of bemcentinib (BGB324), a first-in-class highly selective AXL inhibitor, with pembrolizumab in pts with advanced NSCLC: OS for stage I and preliminary stage II | Journal of Clinical Oncology https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.9098
- Bergenbio Received FDA FAST-TRACK Designation For Bemcentinib / ANTI-PD-(L)1 Combination in NSCLC – https://www.bergenbio.com/bergenbio-received-fda-fast-track- designation-for-bemcentinib-anti-pd-l1-combination-in-nsclc/
- Khobta et al. Ipilimumab: its potential in non-small cell lung cancer. Therapeutic Advances in Medical Oncology March 2012 vol. 4 no. 2 43-50).
- Morgensztern D, Goodgame B, Govindan R. Vaccines and immunotherapy for non-small cell lung cancer. J Thorac Oncol. 2010;5(12 Suppl. 6):S463-S465. Eur J Cancer. 2015 Nov;51(16):2321-9.).
- Harriet Kluger, et al. Phase Ib/II of CD40 agonistic antibody APX005M in combination with nivolumab (nivo) in subjects with metastatic melanoma (M) or non-small cell lung cancer (NSCLC) [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; 2019 Mar 29-Apr 3; Atlanta, GA. Philadelphia (PA): AACR; Cancer Res 2019;79(13 Suppl): Abstract nr CT089.
- Xiubao Ren. Immunosuppressive checkpoint Siglec-15: a vital new piece of the cancer immunotherapy jigsaw Cancer Biol Med 2019. doi: 10.20892/j.issn.2095-3941.2018.0141)
- Garassino MC, et Abstract OA04.06. Presented at: International Association for the Study of Lung Cancer World Conference on Lung Cancer; Sept. 7-10, 2019; Barcelona.
- Socinski M, Velcheti V, Mekhail T, et Final efficacy results from B-F1RST, a prospective phase II trial evaluating blood-based tumour mutation burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Presented at: LBA83. Presented at: European Society for Medical Oncology (ESMO) Congress 2019; September 27-October 1, 2019: Barcelona, Spain.
- Arbour, K. C., Mezquita, L., Long, N., et al. (2018). Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with Non–Small-cell lung cancer. JCO, 36(28), 2872-2878. doi:10.1200/2018.79.0006
- Wakuda, K., Miyawaki, T., Miyawaki, E., et al. (2019). The impact of steroid use on efficacy of immunotherapy among patients with lung cancer who have developed immune-related adverse events. JCO, 37(15), e20583-e20583. doi:10.1200/JCO.2019.37.15_suppl.e20583
- Derosa, L., Hellmann, M. D., Spaziano, M., et al. (2018). Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung Annals of oncology : official journal of the European Society for Medical Oncology, 29(6), 1437–1444. doi:10.1093/annonc/mdy103
- Tien Phuc Do, et al. Antibiotic use and overall survival in lung cancer patients receiving Journal of Clinical Oncology 2018 36:15_suppl, e15109-e15109
- Pinato, D. J., Howlett, S., Ottaviani, D., et al. (2019). Antibiotic treatment prior to immune checkpoint inhibitor therapy as a tumor-agnostic predictive correlate of response in routine clinical practice. JCO, 37(8), 147-147. doi:10.1200/JCO.2019.37.8_suppl.147
- CIMAvax-EGF, a therapeutic non-small cell lung cancer vaccine Expert Opin Biol Ther . 2018 Jul;18(7):829-835. doi: 10.1080/14712598.2018.1492539. Epub 2018 Jul 4.
- A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvax-EGF as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients Clin Cancer Res2016 Aug 1;22(15):3782-90. doi: 10.1158/1078-0432.CCR-15-0855. Epub 2016 Feb 29. – PubMed
- Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B Lancet Oncol. 2011;12(12):1125-33.
- Rochlitz C, Figlin R, Squiban P, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1- positive advanced cancer. J Gene Med. 2003;5(8):690-99.
- Quiox, E et TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomized, double-blind, placebo- controlled, phase 2b/3 trial. The Lancet Volume 17, No. 2, p212–223, February 2016.
- Sebastian, M et al. Phase Ib trial of the RNActive cancer vaccine BI 1361849 (CV9202) and local radiotherapy (RT) in patients (pts) with stage IV NSCLC with disease control after 1st-line chemotherapy or during therapy with an EGFR-TKI: results of an interim J Clin Oncol 34, 2016 (suppl; abstr e20627).
- Alexandros Papachristofilou, et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. Journal for ImmunoTherapy of Cancer volume 7, Article number: 38 (2019))
- Abstract B209: Phase 1/2 study of mRNA vaccine therapy + durvalumab (durva) ± tremelimumab (treme) in patients with metastatic non-small cell lung cancer (NSCLC) | Cancer Immunology Research (aacrjournals.org) https://cancerimmunolres.aacrjournals.org/content/7/2_Supplement/B209
- Kathuria H et Updates and Controversies in the Rapidly Evolving Field of Lung Cancer Screening, Early Detection, and Chemoprevention. Cancer 2014, 6, 1157-1179; doi:10.3390/cancers6021157
- Xu, ; Yin, Z.; Gao, W. et al. Meta-analysis on the association between nonsteroidal anti-inflammatory drug use and lung cancer risk. Clin. Lung Cancer 2012, 13, 44–51, doi:10.1016/j.cllc.2011.06.009).
- Keith, L. (2012). Lung Cancer Chemoprevention. Proceedings of the American Thoracic Society, 9(2), 52–56.)