Lung Cancer Choices© 6th Edition Menu
Chapter 1: Diagnosis and Staging of Lung Cancer
Chapter 2: Comprehensive Biomarker Testing
Chapter 3: Surgery for Lung Cancer Patients
Chapter 5: Radiation Therapy for Non-Small Cell Lung Cancer
Chapter 6: Treatment for Small Cell Lung Cancer
Chapter 7: Clinical Trials and Emerging Therapies for Lung Cancer
Chapter 9: Nutrition in the Patient with Lung Cancer
Chapter 10: Sexuality and Lung Cancer
Chapter 11: Integrative Medicine, Complementary Therapies, and Chinese Medicine in Lung Cancer
Chapter 12: Lung Cancer in People who have Never Smoked
Chapter 13: How to Quit Smoking Confidently and Successfully
Lung Cancer Question Builder - Develop your list of "Questions to Ask" your providers
Lung Cancer in People who have Never Smoked
Jessica A. Hellyer, MD and Heather A. Wakelee, MD

Introduction
Lung cancer is the leading cause of cancer death for both men and women in the United States, and globally it is the leading cause of cancer death in men and the third leading cause in women.1-2 Smoking is the most common cause of lung cancer, but there are many people who have never smoked who develop lung cancer. Lung cancer in people who have never smoked is more common in Asia and Asian populations globally, Hispanic populations and in women.1 The causes of lung cancer in people who have never smoked are not well understood. Lung cancer treatment is similar for patients with early stage disease regardless of whether they smoked. However, for patients with advanced (stage IV) disease, many people who have never smoked who develop lung cancer will have mutations in their tumor which allow the tumor to respond to specific targeted treatments.
Frequency
The information about how many people develop cancer each year comes from cancer registries. These databases compile information about the type of cancer and age of the person, but often lack information about smoking history. Therefore, it is difficult to know how many cases of lung cancer are attributed or caused by tobacco use. Separate registries on smoking patterns are often used to estimate how many people in a population use tobacco. This information can be loosely correlated to the trends in lung cancer incidence from cancer registries to make estimates about how many people with lung cancer have developed the disease without a smoking history. Worldwide, approximately 15% to 20% of men with lung cancer, and 50% of women with lung cancer, are people who have never smoked.1 In the United States, approximately 1 in 10 men, and 1 in 5 women, with lung cancer are people who have never smoked.3
It is unknown whether the incidence of lung cancer is increasing in people who have never smoked. A study in Swedish construction workers who had never smoked showed an increased frequency of lung cancer in the 1990s compared with the 1970s.4 In the United States, more women who had never smoked died of lung cancer in the 1980s and 1990s than in the 1960s.5 Moreover, although lung cancer incidence rates have been declining overall due to the success of tobacco cessation programs, recent data show that lung cancer rates have declined quicker among non-Hispanic men than women, reversing a long-observed trend that more men develop lung cancer than women. Correlation with data from tobacco registries suggests that the relative increase in lung cancer among women in the US is not due to increase in tobacco use.6-7 However, these studies are difficult to do because we do not have smoking information available in the same databases that capture information about the number of patients who develop lung cancer and other reports have shown no increase in lung cancer in people who have never smoked.8-9 There is a sense among doctors who treat lung cancer that the number of people with lung cancer who have never smoked is increasing and this area is being actively investigated. In Taiwan, for example, the majority of patients (53%) diagnosed with lung cancer do not have a smoking history and the type of cancer (adenocarcinoma) and tumor characteristics tend to be different in the lung cancer of people who have never smoked compared to those that have.10 Studies are ongoing to better understand this important issue.
The Causes
The causes of lung cancer in people who have never smoked are unknown, but several factors may increase the risk. (Table 1)11
Environmental exposures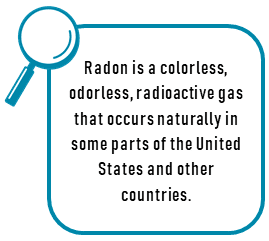 Many environmental toxins have been implicated in increasing the lung cancer risk. Secondhand smoke may cause approximately 20% of the lung cancers in people who have never smoked.12 Air pollution is thought to be responsible for 5% of cases of lung cancer but it may be higher.13 Indoor air pollution, such as fumes from cooking oil and smoke from burning coal, may increase lung cancer risk, especially in Asia.14
Many environmental toxins have been implicated in increasing the lung cancer risk. Secondhand smoke may cause approximately 20% of the lung cancers in people who have never smoked.12 Air pollution is thought to be responsible for 5% of cases of lung cancer but it may be higher.13 Indoor air pollution, such as fumes from cooking oil and smoke from burning coal, may increase lung cancer risk, especially in Asia.14
Radon is a colorless, odorless, radioactive gas that occurs naturally in some parts of the United States and other countries. Some homes have high levels of radon, and this can be tested with home kits. People who live in homes with high levels of radon are at a higher risk of developing lung cancer, whether or not they smoke.15-16 Jobs that expose people to toxic substances, such as uranium, asbestos, chromium, and arsenic, may increase the risk of developing lung cancer.17-18 Arsenic may be present in drinking water in some areas such as Taiwan and Chile.19-20 Nutritional deficiencies may contribute to the development of cancer, and people who eat more fruits and vegetables may be at lower risk for developing lung cancer.21-22
Medical History
Chronic inflammatory diseases of the lung, such as pulmonary fibrosis or interstitial lung disease, can increase the risk of developing lung cancer.23 Lung damage from prior radiation therapy (for example, people who received chest radiation for Hodgkin’s Disease (a type of Lymphoma) or other cancers of the chest) also increases the risk of developing lung cancer.24 In addition, lung cancer risk may be increased in people who hav4e the human papilloma virus, but not everyone agrees with that risk.25 At this time there is no proof that human papilloma virus causes lung cancer.
Genetics
People with family members who have lung cancer have a slightly higher risk of developing lung cancer, but the magnitude and cause of this risk are unknown.11,26-27 Research is being done to try to find what changes in the DNA (genes) may make certain families at higher risk for lung cancer. So far we don’t know any DNA changes that are definitely linked to a higher risk of lung cancer in families and we don’t have a test to help people know if they are at risk. This research is ongoing.
Table 1. Possible Causes of Lung Cancer in People Who Have Never Smoked
| Second hand smoke |
| Radon exposure |
| Other toxins (asbestos, chromium, or arsenic) |
| Dietary Factors (diet deficient in fruits and vegetables) |
| Air pollution (including cooking fumes) |
| Radiation therapy to the chest |
| Other lung diseases such as idiopathic pulmonary fibrosis |
| Human papillomavirus (controversial) |
| Other family members with lung cancer |
| Differences in ability to fix DNA damage |
Characteristics
There are several known differences between lung cancer in smokers and people who have never smoked, including the specific type of cancer. Lung cancer in smokers is often a type called small cell lung cancer or a form of non-small cell lung cancer (NSCLC) known as squamous cell carcinoma. Adenocarcinoma, a different type of NSCLC, is more common in people who have never smoked.1, 28 However, people with a smoking history can also develop adenocarcinoma of the lung and those who have never smoked are rarely diagnosed with squamous cell lung cancer or small cell lung cancer. The only way to know what kind of lung cancer it is for sure is to have a biopsy that is examined by a pathology doctor.
 It is also known that there are racial/ethnic and gender disparities in non-smoking lung cancer. Non-smoking lung cancer is more common in people of Asian ancestry and Hispanics as well as in women.29-31 We know that the percentage of women with lung cancer who have never smoked is higher than the percentage of men with lung cancer who have never smoked, and rising.3, 6 The reason for these differences is not known although theories include a difference in biologic susceptibility to toxins or unequal reduction in workplace exposures.7 Recently we learned more about the risks of lung cancer in Hispanic populations and some evidence of lung cancer susceptibility.32
It is also known that there are racial/ethnic and gender disparities in non-smoking lung cancer. Non-smoking lung cancer is more common in people of Asian ancestry and Hispanics as well as in women.29-31 We know that the percentage of women with lung cancer who have never smoked is higher than the percentage of men with lung cancer who have never smoked, and rising.3, 6 The reason for these differences is not known although theories include a difference in biologic susceptibility to toxins or unequal reduction in workplace exposures.7 Recently we learned more about the risks of lung cancer in Hispanic populations and some evidence of lung cancer susceptibility.32
In about half of the cases of lung cancer in people who have never-smoked, we are able to detect a genetic difference in the tumor which not only caused the cancer, but also serves as a target for therapy. Testing for these DNA changes is now considered standard for patients who have been diagnosed with non-small cell lung cancer, especially adenocarcinoma, to help guide treatment and better understand the disease in each individual. For example, tumors from patients who have never smoked are more likely to have mutations in a protein known as the epidermal growth factor receptor (EGFR).33-34 Tumors with specific changes in the EGFR protein are more likely to shrink when treated with drugs that attack the EGFR protein, such as osimertinib, erlotinib, gefitinib, dacomitinib and afatinib. Another mutation that is more common in the tumors of people with no smoking history is in the Anaplastic Lymphoma Kinase (ALK) gene.35-36 The drugs alectinib, brigatinib, crizotinib, lorlatinib and ceritinib are used to treat lung cancer patients who have the ALK gene rearrangement.
There are other, less common, DNA mutations that are found more frequently in the tumors of patients with non-smoking lung cancer. In addition to EGFR, ALK and ROS1 we now can look for changes in BRAF, RET, MET, HER2 and NTRK and can offer specific “targeted” therapy when identified in tumors of patients with metastatic disease. In general, patients will have only a single major change in their DNA (for example, a patient with a change in the EGFR gene usually does not also have an ALK gene rearrangement). It is important that testing is done to look for EGFR, ALK, ROS1, BRAF and NTRK at a minimum in lung cancer patients who have never-smoked, though there are many other gene changes in tumors of patients who have never smoked which have been identified such as RET, MET and HER2. As more “targeted” treatments are developed it becomes even more important that a tumor is tested for all the mutations that could allow for other treatment options. See Chapter 2: Comprehensive Biomarker Testing
Treatment of Lung Cancer in People Who Have Never Smoked
 Early-Stage Lung Cancer (Stage I-III)
Early-Stage Lung Cancer (Stage I-III)
Treatment for people who have never smoked who have early stage non-small cell lung cancer is the same as treatment for smoking-associated lung cancer. For patients with tumors that can be removed with surgery, surgery is preferred therapy, followed by chemotherapy in some cases (depending on tumor characteristics). For people with stage III lung cancer that cannot be removed with surgery, treatment is a combination of chemotherapy plus radiation therapy, followed by a year of immunotherapy. The latest research now shows that EGFR targeted drugs may help prevent the return of cancer in people early stage lung cancer that has been removed with surgery if the cancer has the EGFR mutation. Recently published data on the use of osimertinib in patients with stage IB-IIIA lung cancer following completion of surgery showed an improvement in the length of time prior to the cancer recurring compared with patients who did not receive osimertinib.37 Though we do not yet know if that approach will change cure rates, the delay in return of cancer is very significant and osimertinib is now a treatment option in this setting.
Advanced Stage Lung Cancer (Stage IV)
People who have never smoked and who have lung cancer are more likely to have changes in specific genes such as EGFR, ALK or ROS1. These gene changes are not seen in the normal cells from a person with lung cancer, only in the cancer cells. In recent years, other genetic changes have been discovered including alterations in BRAF, RET, MET, NTRK, HER2 and others. For most people diagnosed with stage IV non-small cell lung cancer, the first treatment is chemotherapy plus immune therapy or immune therapy alone. Chemotherapy can also work very well for patients with tumors with specific gene mutations; however, if we find the gene mutation before chemotherapy is started we usually start with a drug “targeted” to treat the gene mutation. It is very important for all patients with advanced stage lung cancer, especially patients who have never smoked, to have their tumor tested for genetic changes. This is also called biomarker testing. See Chapter 2: Comprehensive Biomarker Testing
EGFR
Patients with metastatic lung cancer who have specific EGFR gene changes should receive osimertinib as the initial treatment as this has been shown on average to control the tumor longer than chemotherapy and may even lead to living longer compared to starting with other treatments.38 For patients who are unable to receive osimertinib, treatment with erlotinib, gefitinib, dacomitinib or afatinib is preferred over chemotherapy based on studies that have shown that they have a higher chance of shrinking the tumor than chemotherapy for these patients.39-40 In the patients who start on one of the earlier EGFR treatments such as erlotinib, gefitinib, dacomitinib or afatinib, but who have tumor progression, testing for a resistance mutation called T790M is done to see if they would benefit from osimertinib prior to going on to chemotherapy.
The EGFR-targeted drugs are not generally added to chemotherapy, although this is an area of active investigation. A study looking at gefitinib alone versus gefitinib plus chemotherapy showed patients who received gefitinib plus chemotherapy had a longer time on treatment and improvement in overall survival compared with gefitinib alone.41 However, gefitinib is rarely used as first line therapy in the US anymore so it is unclear how to apply this information. Studies looking at osimertinib plus chemotherapy are ongoing. Other studies have looked at whether to add chemotherapy to the targeted drug once it has stopped working. The IMPRESS trial showed that it is not beneficial in the majority of patients to continue the targeted drug when chemotherapy is started when the drug gefitinib was used in this trial.42
Adding other targeted agents to EGFR drugs has also been examined. For example, bevacizumab, a drug that reduces blood vessel formation in tumors, was investigated in combination with erlotinib. On average, patients who received both drugs together had their tumor controlled almost twice as long as patients who received erlotinib alone. Despite this, there was no difference between in the two groups in how long patients lived with cancer.43 Another blood vessel targeted therapy, ramucirumab, was also looked at in combination with erlotinib and showed that when given together patients had longer tumor control compared to erlotinib alone. Based off of this data, this is a reasonable initial treatment option, although we are still waiting to see whether this combination will result in an increase in survival.44 Trials looking at osimertinib in combination with bevacizumab are underway although the preliminary data from a study looking at osimertinib in combination with bevacizumab in patients with a tumor containing a specific EGFR mutation (T790M) failed to show a benefit.45 Most of the information above is for the most common mutations in EGFR (the del19 and L858R mutations). There are other less common EGFR mutations that are treated similarly. However other mutations in EGFR, especially those known as “exon20” mutations make tumors less likely to respond and newer treatments are being developed.
ALK
There are many targeted drug options for patients who have the ALK gene rearrangement (most common in lung cancer patients who have never smoked). Crizotinib was the only clinically available ALK+ targeted agent for several years but is no longer the preferred front line therapy. There are three recommended options for first line treatment of ALK+ lung cancer. First, is alectinib based off data from the ALEX trial showing improved disease control (almost three times longer) on alectinib compared to crizotinib and found that alectinib had fewer side effects.46 In addition, alectinib does a better job of controlling and preventing cancer metastases to the brain compared with crizotinib. Other options for front line treatment include brigatinib based off of data that showed that brigatinib did a better job controlling tumors (both in and out of the brain) than crizotinib.47 Lorlatinib was the most recent agent to be approved as a front line option after results from the CROWN trial showed a doubling of the number of patients alive and without disease progression in the group that was treated with lorlatinib compared with crizotinib.48 Finally, Certinib, an older ALK targeted agent is also an option for front line treatment. Ceritinib has not been compared to crizotinib, but was studied against chemotherapy in the ASCEND-4 study that showed that ceritinib did a better job of controlling disease compared with chemotherapy.49
After progression, treatment with another ALK targeted agent is preferred prior to chemotherapy. Options in the second line setting depend in part on which drug was given first and is tailored to each patient. Alectinib is the most common drug used in this setting, but the other ALK drugs may also be appropriate depending on each patient’s treatment history.
ROS1
ROS1 rearrangements are also more common in patients who have never smoked. In early studies of crizotinib in patients with previously treated ROS1 positive lung cancer, the majority of patients had their tumor respond to crizotinib and this response lasted on average about a year and a half. For this reason, crizotinib is approved as a first treatment of ROS1 lung cancer.50 More recently, entrectinib was also approved for up front treatment of ROS1 positive lung cancer. This was based off information from three small studies that showed that about three quarters of patients treated with entrectinib had a response in their tumor. Importantly, entrectinib also seems to work well in patients whose lung cancer has grown in the brain.51 Ceritinib also has some activity in ROS1 mutant tumors and is another option for first line treatment.52 There are multiple options when the first line treatment stops working and this includes other ROS1 “targeted” drugs, chemotherapy or clinical trial.
Other genetic changes
In addition to EGFR, ALK and ROS1 we now can look for changes in BRAF, RET, MET, NTRK and HER2 and can offer specific “targeted” therapy when identified in tumors of patients with metastatic disease. BRAF inhibitors approved for use in lung cancer are the combination of dabrafenib/trametinib. HER2 active drugs include TDM-1, afatinib and trastuzumab. Multiple drugs are active for RET including selpercatinib, pralsetinib, cabozantinib and vandetinib. Cabozantinib is also active for METexon 14 splice site variations, as are tepotinib, capmatinib and crizotinib. Larotrectinib and entrectinib were both recently approved for treatment of tumors with NTRK fusions.
Chemotherapy and Immune therapy One theory as to how cancer is able to develop and grow is that it acquires mechanisms to evade the body’s immune system. Immune therapy is a cancer treatment designed to teach the body to recognize tumor as foreign and attack it. Those drugs have been shown to work in lung cancer regardless of smoking history, but perhaps less so in patients with no smoking history. Patients with mutations in EGFR or ALK were excluded from many of the early immune therapy trials, so it is unknown how well this group benefits from immune therapy and there is some evidence to suggest their tumors do not respond as well. Efforts to get immune therapy to work in patients with limited smoking history are ongoing with multiple combination drug studies. For patients without targetable mutations, preferred first line treatment is a combination of chemotherapy plus immunotherapy. This has been shown to work in some patients regardless of smoking status.53
One theory as to how cancer is able to develop and grow is that it acquires mechanisms to evade the body’s immune system. Immune therapy is a cancer treatment designed to teach the body to recognize tumor as foreign and attack it. Those drugs have been shown to work in lung cancer regardless of smoking history, but perhaps less so in patients with no smoking history. Patients with mutations in EGFR or ALK were excluded from many of the early immune therapy trials, so it is unknown how well this group benefits from immune therapy and there is some evidence to suggest their tumors do not respond as well. Efforts to get immune therapy to work in patients with limited smoking history are ongoing with multiple combination drug studies. For patients without targetable mutations, preferred first line treatment is a combination of chemotherapy plus immunotherapy. This has been shown to work in some patients regardless of smoking status.53
For patients with mutations in EGFR or ALK who have had growth of their cancer on targeted therapy, a 4-drug combination of chemotherapy plus bevacizumab (target against blood vessels) plus immunotherapy was found to have a small benefit in tumor control and the suggestion of possible improvement in survival.54 However, the number of patients in the study with EGFR or ALK mutations who received this combination was small (111 patients) so additional data is needed to understand how to best use these treatments in patients who develop molecularly targeted mutations. Chemotherapy alone is another good option for patients with non-smoking lung cancer that has grown on targeted therapy (in the case of having a targetable mutation) or on a chemo-immunotherapy combination. The combination of targeted therapy plus immunotherapy has been examined in a number of trials but unfortunately this combination has not been very effective and notably has a high rate of toxicity.55 The most important point is that the best treatment cannot be chosen until all the information about a tumor is known. The results for immune therapy (PD-L1) often come back sooner than the results about EGFR, ALK and the other potential tumor mutations. But when lung cancer develops in someone with a limited history of smoking the chance of a tumor having a mutation that can be treated is very high. So it is critical to wait to get the tumor mutation results back before starting treatment, unless there is a medical reason that treatment must start sooner. Starting immune therapy (even with chemotherapy) can increase the risk of toxicity from some of the targeted treatments (like osimertinib), which can be a problem if that treatment is started before knowing about the tumor mutations.
Conclusions
 Lung cancer can happen to anybody, whether or not that person has ever smoked. Although lung cancer is very similar in patients whether or not they have a history of smoking, there are some differences. These include the types of people with the disease (lung cancer in people who have never smoked happens more often in women and in people from different ethnic groups including Asian or Hispanic. It is also seen more often if someone develops cancer very young. When lung cancer develops in someone who has never smoked it is more likely to be the adenocarcinoma type.
Lung cancer can happen to anybody, whether or not that person has ever smoked. Although lung cancer is very similar in patients whether or not they have a history of smoking, there are some differences. These include the types of people with the disease (lung cancer in people who have never smoked happens more often in women and in people from different ethnic groups including Asian or Hispanic. It is also seen more often if someone develops cancer very young. When lung cancer develops in someone who has never smoked it is more likely to be the adenocarcinoma type.
Some causes of lung cancer other than smoking have been identified, including second-hand smoke, radon exposure, cooking fumes, family history, and others. We know that patients with the disease who have never smoked are more likely to have tumors with mutations in the EGFR gene, ALK gene rearrangements or other gene changes in the tumor that can change treatment plans. Patients who have specific EGFR gene changes have a better response to EGFR blocking drugs like osimertinib or erlotinib, and patients with the ALK gene rearrangement usually respond well to alectinib, brigatinib or ceritinib. Further research will provide more information about the cause of this type of lung cancer and how to best treat patients with this illness. People who want to know more about this topic can look at recent reviews that have been written for doctors.28,56
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
- Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472-478.
- Boffetta P, Järvholm B, Brennan P, Nyrén O. Incidence of lung cancer in a large cohort of non-smoking men from Sweden. Int J Cancer. 2001;94(4):591-593.
- Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst. 2006;98(10):691-699.
- Jemal A, Miller KD, Ma J, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med. 2018;378(21):1999-2009.
- Hellyer JA, Patel MI. Sex disparities in lung cancer incidence: validation of along-observed trend. In. Transl Lung Cancer Res2019.
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. 2000;321(7257):323-329.
- Kawaguchi T, Matsumura A, Fukai S, et al. Japanese ethnicity compared with Caucasian ethnicity and never-smoking status are independent favorable prognostic factors for overall survival in non-small cell lung cancer: a collaborative epidemiologic study of the National Hospital Organization Study Group for Lung Cancer (NHSGLC) in Japan and a Southern California Regional Cancer Registry databases. J Thorac Oncol. 2010;5(7):1001-1010.
- Yang P-C. National Lung Cancer Screening Program in Taiwan: The TALENT Study. January 26-31, 2021. World Conference on Lung Cancer Singapore.
- Brenner DR, Hung RJ, Tsao MS, et al. Lung cancer risk in never-smokers: a population-based case-control study of epidemiologic risk factors. BMC Cancer. 2010;10:285.
- Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96(2):99-106.
- Vineis P, Hoek G, Krzyzanowski M, et al. Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health. 2007;6:7.
- Kleinerman RA, Wang Z, Wang L, et al. Lung cancer and indoor exposure to coal and biomass in rural China. J Occup Environ Med. 2002;44(4):338-344.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A. 2006;69(7):533-597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. 2005;330(7485):223.
- Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23(14):3175-3185.
- Neuberger JS, Field RW. Occupation and lung cancer in nonsmokers. Rev Environ Health. 2003;18(4):251-267.
- Chen CL, Hsu LI, Chiou HY, et al. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. 2004;292(24):2984-2990.
- Ferreccio C, González C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. 2000;11(6):673-679.
- Wakai K, Ando M, Ozasa K, et al. Updated information on risk factors for lung cancer: findings from the JACC Study. J Epidemiol. 2005;15 Suppl 2:S134-139.
- Gorlova OY, Weng SF, Hernandez L, Spitz MR, Forman MR. Dietary patterns affect lung cancer risk in never smokers. Nutr Cancer. 2011;63(6):842-849.
- Karampitsakos T, Tzilas V, Tringidou R, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2017;45:1-10.
- Hoskin PJ, Smith P, Maughan TS, et al. Long-term results of a randomised trial of involved field radiotherapy vs extended field radiotherapy in stage I and II Hodgkin lymphoma. Clin Oncol (R Coll Radiol). 2005;17(1):47-53.
- Cheng YW, Chiou HL, Sheu GT, et al. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 2001;61(7):2799-2803.
- Wu AH, Fontham ET, Reynolds P, et al. Family history of cancer and risk of lung cancer among lifetime nonsmoking women in the United States. Am J Epidemiol. 1996;143(6):535-542.
- Bailey-Wilson JE, Amos CI, Pinney SM, et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet. 2004;75(3):460-474.
- Subramanian J, Govindan R. Lung cancer in ‘Never-smokers’: a unique entity. Oncology (Williston Park). 2010;24(1):29-35.
- Epplein M, Schwartz SM, Potter JD, Weiss NS. Smoking-adjusted lung cancer incidence among Asian-Americans (United States). Cancer Causes Control. 2005;16(9):1085-1090.
- Gomez SL, Chang ET, Shema SJ, et al. Survival following non-small cell lung cancer among Asian/Pacific Islander, Latina, and Non-Hispanic white women who have never smoked. Cancer Epidemiol Biomarkers Prev. 2011;20(3):545-554.
- Ou S, Ziogas A, Zell J. Epidemiology study of never-smokers with non-small cell lung cancer (NSCLC): High percentages of Asian and Hispanic female never-smokers and the significance of Asian ethnicity. In. Vol 26. J Clin Onc. 2008:8004.
- Carrot-Zhang J, Soca-Chafre G, Patterson N, et al. Genetic Ancestry Contributes to Somatic Mutations in Lung Cancers from Admixed Latin American Populations. Cancer Discov. Mar 2021;11(3):591-598. doi:10.1158/2159-8290.CD-20-1165
- Sonobe M, Manabe T, Wada H, Tanaka F. Mutations in the epidermal growth factor receptor gene are linked to smoking-independent, lung adenocarcinoma. Br J Cancer. 2005;93(3):355-363.
- Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12(5):1647-1653.
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15(16):5216-5223.
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 10 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113-125.
- Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
- Noronha V, Joshi A, Patil VM, Chougule A, Mahajan A, al. e. Phase III randomized trial comparing gefitinib to gefitinib with pemetrexed-carboplatin chemotherapy in patients with advanced untreated EGFR mutant non-small cell lung cancer (gef vs gef+C). In. Vol 37. J Clin Oncol. 2019.
- Mok TSK, Kim SW, Wu YL, et al. Gefitinib Plus Chemotherapy Versus Chemotherapy in Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer Resistant to First-Line Gefitinib (IMPRESS): Overall Survival and Biomarker Analyses. J Clin Oncol. 2017;35(36):4027-4034.
- Yamamoto N, Seto T, Nishio M, Goto K, Okamoto I, al e. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutation–positive non-squamous non–small-cell lung cancer (NSCLC): Survival follow-up results of JO25567. In. Vol 36. J Clin Oncol. 2018:9007.
- Nakagawa K, Garon EB, Seto T, al e. RELAY: A multinational, double-blind, randomized Phase 3 study of erlotinib (ERL) in combination with ramucirumab (RAM) or placebo (PL) in previously untreated patients with epidermal growth factor receptor mutation-positive (EGFRm) metastatic non-small cell lung cancer (NSCLC). In. Vol 37. J Clin Oncol. 2019:9000.
- Akamatsu H, Toi Y, Hayashi H, et al. Efficacy of Osimertinib Plus Bevacizumab vs Osimertinib in Patients With EGFR T790M-Mutated Non-Small Cell Lung Cancer Previously Treated With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: West Japan Oncology Group 8715L Phase 2 Randomized Clinical Trial. JAMA Oncol. Mar 2021;7(3):386-394. doi:10.1001/jamaoncol.2020.6758
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. 2017;390(10089):29-39.
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2027-2039.
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced. N Engl J Med. 11 2020;383(21):2018-2029. doi:10.1056/NEJMoa2027187
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 03 2017;389(10072):917-929. doi:10.1016/S0140-6736(17)30123-X
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. Nov 2014;371(21):1963-71. doi:10.1056/NEJMoa1406766
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 02 2020;21(2):261-270. doi:10.1016/S1470-2045(19)30690-4
- Lim SM, Kim HR, Lee JS, et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol. 2017;35(23):2613-2618.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078-2092.
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387-401.
- Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf. 2017;16(4):465-469.
- Rudin CM, Avila-Tang E, Harris CC, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res. 2009;15(18):5646-5661.