Lung Cancer Choices© 6th Edition Menu
Chapter 1: Diagnosis and Staging of Lung Cancer
Chapter 2: Comprehensive Biomarker Testing
Chapter 3: Surgery for Lung Cancer Patients
Chapter 5: Radiation Therapy for Non-Small Cell Lung Cancer
Chapter 6: Treatment for Small Cell Lung Cancer
Chapter 7: Clinical Trials and Emerging Therapies for Lung Cancer
Chapter 9: Nutrition in the Patient with Lung Cancer
Chapter 10: Sexuality and Lung Cancer
Chapter 11: Integrative Medicine, Complementary Therapies, and Chinese Medicine in Lung Cancer
Chapter 12: Lung Cancer in People who have Never Smoked
Chapter 13: How to Quit Smoking Confidently and Successfully
Lung Cancer Question Builder - Develop your list of "Questions to Ask" your providers
Diagnosis and Staging of Lung Cancer
Tze-Ming Chen, MD, FCCP
 Introduction
Introduction
Lung cancer remains the leading cause of cancer-related mortality in the United States despite advances in chemotherapeutic options and surgical techniques. The evaluation of patients with suspected or known lung cancer requires accurate and preferably rapid diagnosis and staging to guide the optimal treatment regimen: surgical resection, surgical resection with adjuvant chemotherapy, chemotherapy alone, chemotherapy in conjunction with radiation therapy, or use of targeted therapies. Currently, staging may include combined positron emission tomography – computed tomography (PET-CT) imaging, endobronchial ultrasound guided-fine needle aspiration (EBUS-FNA), endoscopic ultrasound guided-FNA (EUS-FNA), guidance assisted bronchoscopy, mediastinoscopy, thoracentesis, pleuroscopy, video-assisted thoracoscopic surgery (VATS), and or computed tomography (CT) or ultrasound guided FNA.
This chapter will review the current system for staging non-small cell lung cancer (NSCLC), the different diagnostic and staging options, and a brief discussion about the importance of mutation analyses in guiding treatment for patients with advanced stage disease. I will then provide a summary of our center’s approach towards lung cancer diagnosis and staging with supporting literature where available.
Staging Background
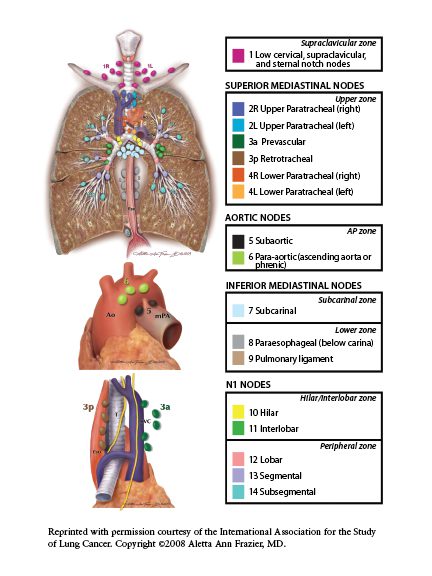
The 8th Edition of the American Joint Commission on Cancer TMN staging system for NSCLC which became the worldwide standard in 2017 continues with the existing method of assessing tumor size and its effect on the surrounding lung tissue or its interaction with non-lung tissue (T), the extent of spread of lung cancer to lymph nodes (N) and the presence or absence of metastatic spread of lung cancer outside of lung tissue or to the contralateral lung (M)1 (Figure 1).2 The TNM classification system is then used to derive a stage of NSCLC which ranges from localized disease (IA) to wide-spread disease (IV) providing information on expected prognosis and survival.
Figure 1: Schematic of the lymph node stations within the chest2
Diagnostic and Staging Modalities
Combined PET-CT
PET is an imaging technique that captures the level of metabolic activity of different tissues. Patients are given an intravenous injection of 2-(18F) fluoro-2-deoxy-D- glucose (FDG) followed by imaging 60 minutes later. The degree of metabolic activity correlates with the level of FDG uptake which is reported as a standardized uptake value (SUV). A number of studies have demonstrated the accuracy of PET for the diagnosis of lung cancer in pulmonary nodules and masses as well as for staging evaluation.3-4 In 2009, a study demonstrated that combined PET-CT improves the selection of patients with known or suspected lung cancer for surgery by decreasing the number of patients with advanced stage lung cancer undergoing surgery.5 A subsequent retrospective study evaluating patients with clinical stage IA NSCLC found PET-CT to have a sensitivity of 44%, specificity of 83%, positive predictive value of 78%, and negative predictive value of 91% compared to surgery for detection of mediastinal lymph node metastases.6
More recently, a team reviewed 64,103 veterans diagnosed with NSCLC between September 2000 and December 2013 and found that PET-CT use increased over time and its use improved stage appropriate therapy for all stages of NSCLC while improving mortality in a risk-adjusted model.7 With lung cancer screening detected lung nodules, PET-CT has a role in selected patients to aid in the risk stratification of indeterminate lung nodules measuring greater than 10 mm in diameter or with evidence of growth if the lesion is less than 10 mm.8
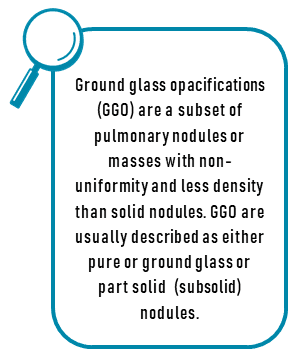 We now have more clarity when deciding which patients should undergo invasive mediastinal staging in the setting of negative PET-CT results. Research in 2017 evaluated 284 consecutive patients with PET- CT staged T1 to 2, N0 NSCLC who then underwent either endobronchial ultrasound or mediastinoscopy sampling of mediastinal lymph nodes. 7% of these patients were found to have occult (hidden) N2 disease with PET-CT and EBUS / mediastinoscopy having a negative predictive value of 92.9% and 96.3%, respectively. In addition, occult N2 disease was more likely to be present in patients with T2 disease compared with those with T1 disease (11.8% compared with 3.6%, p=0.009). Pure solid lesions were also more likely to have N2 disease compared to tumors with any ground glass (12.6% compared to 3.1%, P < 0.001) and patients with central tumors were more likely to have occult N2 disease compared to patients with peripheral lesions (17.5% compared with 4.4%, p < 0.001). Consequently, the authors recommend invasive mediastinal staging for patients with central and solid tumors while those with peripheral ground glass lesions may not warrant such an approach.9
We now have more clarity when deciding which patients should undergo invasive mediastinal staging in the setting of negative PET-CT results. Research in 2017 evaluated 284 consecutive patients with PET- CT staged T1 to 2, N0 NSCLC who then underwent either endobronchial ultrasound or mediastinoscopy sampling of mediastinal lymph nodes. 7% of these patients were found to have occult (hidden) N2 disease with PET-CT and EBUS / mediastinoscopy having a negative predictive value of 92.9% and 96.3%, respectively. In addition, occult N2 disease was more likely to be present in patients with T2 disease compared with those with T1 disease (11.8% compared with 3.6%, p=0.009). Pure solid lesions were also more likely to have N2 disease compared to tumors with any ground glass (12.6% compared to 3.1%, P < 0.001) and patients with central tumors were more likely to have occult N2 disease compared to patients with peripheral lesions (17.5% compared with 4.4%, p < 0.001). Consequently, the authors recommend invasive mediastinal staging for patients with central and solid tumors while those with peripheral ground glass lesions may not warrant such an approach.9
Detecting brain metastases is challenging with PET-CT as FDG is avidly taken up by brain tissue. One retrospective study suggests this imaging modality may assist in selecting patients for brain MRI with a sensitivity of 72% and specificity of 100%.10
It is important to realize that FDG uptake also occurs in inflammatory and infectious processes thereby limiting its ability to discriminate between these processes and cancers.11 Therefore, it is important to obtain tissue confirmation of cancer for FDG-avid lesions.
False negatives can result from the limited spatial resolution of PET scanners affecting the accuracy of this test in subcentimeter lung nodules as well as small lymph nodes.12 In addition, some lung cancers such as bronchioloalveolar carcinomas and carcinoid tumors have been reported to have negative PET imaging results.13 Patients with poorly controlled diabetes mellitus or high blood glucose levels are also more likely to have false negative studies as a result of the elevated levels of endogenous glucose competing for uptake with FDG.
Cancers with low or negative PET signal appear to be associated with better prognoses.14 In addition, the change in activity with chemotherapy correlates with histopathologic response.15
Re-evaluation by PET/CT after neoadjuvant chemotherapy in a 2016 retrospective study of 17 patients with NSCLC with N2 lymph node involvement demonstrated a sensitivity of 100% and specificity of 94%.16
Endobronchial Ultrasound-Guided Fine Needle Aspiration (EBUS-FNA)
EBUS-FNA is a minimally invasive technique that complements mediastinoscopy by its ability to access lymph node stations 2, 3, 4, 7, 10, and 11 (Table 1).
Table 1.
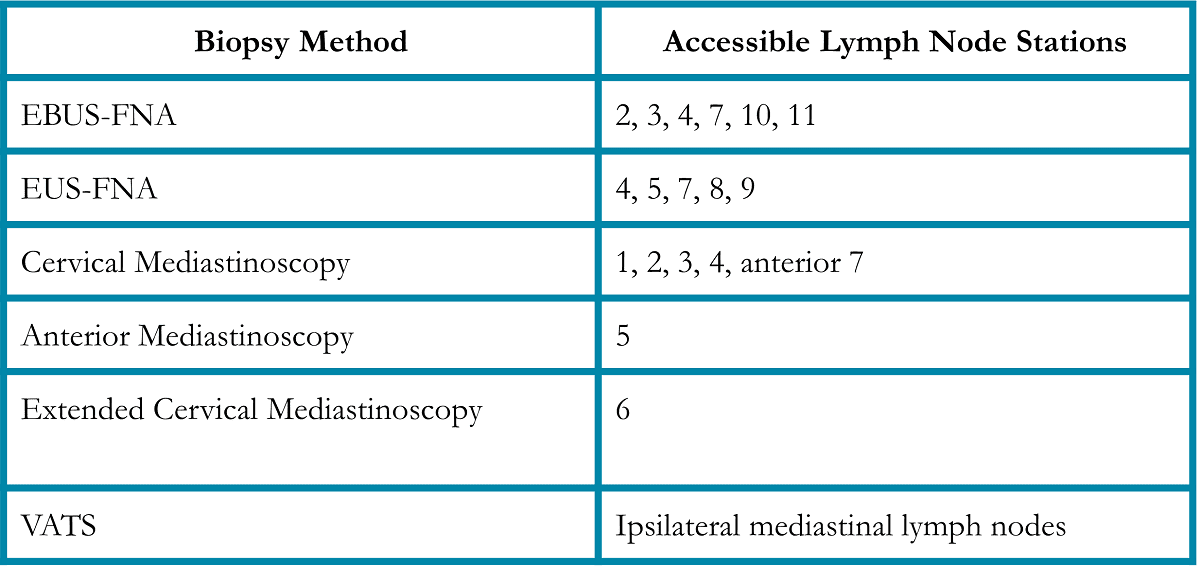
EBUS is a bronchoscopic technique that utilizes ultrasound to identify and permit real-time ultrasound-guided needle biopsy of paratracheal, hilar, and interlobar lymph nodes. In 2003, a group reported their initial experience with EBUS-FNA of mediastinal and hilar lesions under general anesthesia.17 These investigators reported accurate sampling of lymph nodes from stations 1, 2, 4, 7, and 10, with 9 diagnoses of malignancy and 2 diagnoses of benign disease. Subsequent studies have demonstrated that EBUS-FNA is a minimally invasive, highly accurate alternative as well as a complementary procedure to mediastinoscopy for mediastinal and hilar staging for patients with known or suspected NSCLC.18-24
Endoscopic Ultrasound-guided Fine Needle Aspiration (EUS-FNA)
EUS is an additional minimally invasive ultrasound- based technique which uses esophagogastro-endoscopy to sample para-esophageal lymph nodes. These include paratracheal (station 4), aortopulmonary window (station 5), posterior subcarinal (station 7), paraesophageal (station 8), and pulmonary ligament (station 9) lymph nodes (Table 1). Consequently, this technique complements both mediastinoscopy and EBUS-FNA with the additional advantage of being able to access stations 8 and 9 as well as subdiaphragmatic structures including the celiac nodes and the adrenal glands.
Studies evaluating EUS-FNA for lung cancer, excluding one, have demonstrated sensitivities and specificities for malignancy of 87% – 96% and 100%, respectively which is comparable to EBUS- FNA. Overall, these studies demonstrate that EUS is a valuable diagnostic and staging tool for patients with suspected or known NSCLC.25-26
Guidance-assisted Bronchoscopy
One of the most significant limitations to using bronchoscopy for the diagnosis of early stage lung cancer is the inaccuracy of bronchoscopy directed biopsy of lung nodules. Electromagnetic navigation is able to overcome this limitation for selecting lesions that are more than 1 to 1.5 cm in diameter. This system marries CT imaging with bronchoscopy allowing the physician to determine the position of the bronchoscope and a special guidance catheter within the lung of a patient. By performing pre- procedural planning, the physician is now able to maneuver a guidance catheter through a patient’s airways to biopsy lung nodules that are concerning for cancer. In addition, the system allows the placement of fiducial markers around the lung nodule to facilitate treatment with stereotactic radiation.
The major limitations to the success of the procedure include:
-
- the patient’s ability to tolerate bronchoscopy and its associated sedation,
- the size of the lesion of interest as well as its location,
- the experience of the physician performing the procedure, and,
- the actual biopsy is not performed under real-time visualization of the target.
In addition, this procedure is not recommended for patients who have an implanted cardioverter defibrillator or pacemaker due to potential interference between these devices and the electromagnetic field created by the bronchoscopy system.
Risks of the procedure include pain, bleeding, or collapsed lung. However, these risks occur less frequently when compared to CT-guided biopsy or CT-guided placement of fiducial markers.
In early 2018, a new guidance bronchoscopy system was approved by the FDA, robotic bronchoscopy. This new technology provides greater maneuverability within patient airways improving our ability to reach peripheral lung nodules as well as improved stabilization of bronchoscope and biopsy tools within these airways once the target has been reached with improvement in the feasibility and accuracy of lung nodule biopsies. A study in 2020, described a comparison of successful sampling of artificially produced peripheral lung nodules in cadavers between radial endobronchial ultrasound bronchoscopy, electromagnetic navigational bronchoscopy, and robotic bronchoscopy. These investigators found that robotic bronchoscopy had the highest rate of successful biopsy with the lowest rate of missed biopsy. With the robotic system, 10% of attempts sampled the center of the lesion, 65% within the periphery of the lesion, 10% passed through the nodule with the tip of the needle on the other side, 10% had the biopsy needle just outside of the lesion, and 10% of the biopsies missed the nodule. There were no situations where the robotic system was unable to localize the lesion, whereas 35% of the radial endobronchial ultrasound bronchoscopy and 15% of the electromagnetic navigational bronchoscopy biopsy attempts failed to localize the lesion.27
A more recent study in 2021 evaluated the feasibility and safety of robotic bronchoscopy in localizing peripheral lung nodules in patients in a prospective multicenter trial. 54 patients consented and underwent robotic bronchoscopic localization and biopsy of peripheral lesions with a median diameter of 23 mm. 32 of these nodules were in contact with a visible airway on CT imaging. A diagnosis was made in 40 patients (74.1%) with malignancy being the etiology in 33 patients. If the lesion surrounded the airway, the diagnostic yield was 80.6%. If the lesion was adjacent to but not surrounding the airway, the diagnostic yield dropped to 70%. Importantly, pneumothorax complicated robotic bronchoscopy in 2 (3.7%) of the 54 cases, one of which required a chest tube (1.9%).28
As this is a new technology, additional trials are being conducted evaluating the clinical utility of robotic bronchoscopy in the evaluation of peripheral lung lesions including the PRECIsE and TARGET trials.29-30 Currently, multiple centers across the United States have adopted this technology and are actively utilizing it to assist in the work-up of possible lung cancer lesions.
Radial Endobronchial Ultrasound Bronchoscopy (rEBUS)
An adjunctive diagnostic device is the radial endoscopic ultrasound. This device can be inserted through a standard bronchoscope and maneuvered into the lung tissue to help localize a nodule for biopsy. The major limitation with this modality is that the bronchoscopist must maneuver the probe to the nodule with studies suggesting that in 6 to 31% of cases, the lung nodule could not be visualized.31-34 In addition, as with the electromagnetic navigational bronchoscopy system, the limitation of this technique is that the ultrasound probe is then removed so that a biopsy catheter can be inserted. Consequently, the biopsy is not under real-time visualization of the target.
In current practice, a guidance system is used to maneuver a working channel to the lesion of interest followed by confirmation that the catheter is in the correct position using the radial ultrasound. Because the ultrasound probe is removed to allow insertion of the biopsy catheter, the operator is still left without real-time imaging during the actual biopsy.35
Cervical and Anterior Mediastinoscopy
Mediastinoscopy involves an incision at the base of the neck just above the suprasternal notch, followed by the insertion of a mediastinoscope along the length of the trachea to permit sampling of the paratracheal lymph nodes (stations 1, 2, 3, and 4) as well as anterior subcarinal lymph nodes (Table 1). An extended cervical mediastinoscopy allows access to the para- aortic lymph nodes (station 6). The video mediastinoscope permits easier handling and visualization during the procedure as well as potential access to posterior subcarinal lymph nodes.36-37
The major limitations to performing mediastinoscopy are bleeding disorders, severe kyphosis, contraindications to general anesthesia, tracheostomy, or previous chest or neck radiation. The scarring and fibrosis associated with radiation or prior procedures significantly increases the risk of damage to mediastinal organs and vasculature during attempted blunt dissection with the mediastinoscope.
Anterior mediastinoscopy (Chamberlain procedure) permits the evaluation of the aortopulmonary window lymph nodes (Table 1). This involves an incision at the level of the 2nd or 3rd intercostal space to the left of the sternum and the placement of a mediastinoscope to visualize and biopsy visible lymph nodes. The procedure has not been extensively studied but 2 studies have reported false negative rates of 0% and 11%.38-39 It is generally well tolerated, and most patients can avoid an overnight hospital stay.40
Thoracentesis
Patients with pleural effusions that layer at least 1 cm on lateral decubitus chest radiographs are easily assessed for malignancy by thoracentesis. If the fluid is trapped in pockets in the pleural space or loculated, then ultrasound guidance can permit safe sampling of the fluid. This procedure requires only local anesthesia with 1% lidocaine and the placement of a temporary drainage catheter to remove the available pleural fluid. The procedure can be performed in an outpatient setting and is generally well tolerated by the patient. One often discussed complication is lung collapse also referred to as pneumothorax. A prospective study of 506 thoracenteses in 370 patients reported 18 (4%) pneumothoraces.41 Additional complications include catheter insertion site pain, coughing, hemothorax, localized infection, intraabdominal organ injury, and post-expansion pulmonary edema. Contraindications to performing thoracentesis include bleeding disorders unless reversible, infection or abscess of the overlying skin, and the inability to localize a pocket of fluid for sampling.
Pleural fluid analysis will obtain a diagnosis of metastatic adenocarcinoma in 70% of cases but only 20% of squamous cell carcinomas will be detected this way.42 The rate of detection is dependent upon the type of carcinoma, the number of pleural fluid specimens obtained, and the extent of pleural involvement.43
Medical Thoracoscopy (Pleuroscopy)
Pleuroscopy is a procedure that allows access to the pleural space (the potential space between the inner surface of the chest wall and the outer surface of the lung) using an endoscope. In the setting of a diagnosis of or suspicion for lung cancer with a large pleural effusion, pleuroscopy can allow removal of pleural fluid for diagnosis and staging while also potentially relieving shortness of breath or chest discomfort related to the presence of the fluid. In addition, if there are suspicious lesions seen on CT imaging or during the procedure along the inner chest wall lining, biopsies of these lesions can be done. Studies have demonstrated a high diagnostic yield for pleural effusions.44-46
If the pleural effusion is refractory and causing symptoms as described above, medical thoracoscopy can also assist with pleurodesis. These interventions can also be performed during video-assisted thoracoscopic surgery as described in the next section of this chapter but a medical thoracoscopy is usually more limited in its scope and more easily tolerated. It is usually performed by a pulmonologist under conscious sedation with local anesthesia or the patient can undergo general anesthesia. Successful pleurodesis reported in recent publications ranges between 78% and 88% for malignant effusions with a lower success rate for malignant effusions related to lung cancer (72.3%).47-48
Complications of medical pleuroscopy include pain usually from the chest tube placed at the end of the procedure, infection, bleeding, a persistent air leak resulting from a tear of the visceral pleural surface, or subcutaneous emphysema which is the result of air entering the subcutaneous tissue.
More serious but rare complications include death, development of fluid in the lung when it re- expands after the fluid has been removed, or air entering the bloodstream. If cancer is present in the pleural space, there is also a risk of seeding the surgical site with tumor resulting in tumor growth through the site. This final complication is more common with mesothelioma.
In summary, medical thoracoscopy is a diagnostic option for pleural effusions that remain undiagnosed despite thoracentesis as well as a therapeutic approach for pleurodesis in refractory pleural effusions.
Video-Assisted Thoracoscopic Surgery
VATS or thoracoscopy is a surgical method that permits the surgeon to evaluate the pleural space and ipsilateral lymph nodes and to resect lung cancer. The procedure requires general anesthesia, single lung ventilation, and usually a short hospital stay. It is usually well tolerated with an average complication rate of 2%.49 The most common complication is prolonged air leaks.
An important application of VATS is to directly visualize tumors that are radiographically staged T4. Several studies support the use of VATS to confirm T4 lesions designated by CT prior to categorizing the cancer as unresectable.50-51 Thoracoscopy can also evaluate the pleural space for malignancy in patients with pleural effusions that are cytologically negative on repeated thoracentesis or in patients with pleural abnormalities detected on CT. In addition, VATS provide an alternative approach to anterior and extended cervical mediastinoscopy for the evaluation of lymph node stations 5 and 6, respectively (Table 1).
Computed Tomography or Ultrasonography Guided Fine Needle Aspiration
Patients with suspected or known NSCLC who are found to have extra-thoracic disease on PET-CT imaging should undergo tissue biopsy to confirm a metastatic focus. This can be achieved using CT-guided or ultrasound-guided fine-needle aspiration. The procedure is generally very well tolerated and can be performed in an outpatient setting.
Targetable Mutations in Lung Cancer
The diagnostic evaluation of a patient with suspected lung cancer in the early 21st century includes 3 specific goals:
- Does the patient have lung cancer and if so, what type of lung cancer is present?
- What is the pathologic stage of lung cancer?
- If appropriate, are specific mutations present in the lung cancer that could be targeted by a specific therapy?
Using the techniques described above, the ideal for an individual patient would be to achieve these 3 goals in a single procedural setting. Currently, this is possible but as the number of targetable mutations increases, we may reach a point where a separate diagnostic procedure is performed to obtain enough tissue for all the testing needed to determine the most appropriate first-line treatment.
Currently, the National Comprehensive Cancer Network (NCCN) recommends testing patients with advanced stage NSCLC for gene alterations to identify potentially effective therapy targets.52 The potential targets include but are not limited to epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), B-Raf proto-oncogene (BRAF), KRAS proto-oncogene (KRAS), programmed death ligand 1 (PD-L1), MET proto- oncogene (MET), RET proto-oncogene (RET), erythroblastic oncogene B (ERBB2) also frequently called human epidermal growth factor receptor 2 (HER2), and tumor mutational burden (TMB). Consequently, we attempt to obtain as much tumor tissue as possible to send for a broad panel- based molecular profiling test to identify whether an actionable mutation is present.
If the patient is too ill or at too high risk for serious complications to undergo tissue sampling to test for these mutations, a peripheral blood sample also referred to as a liquid biopsy could be used to try to assess for these gene alterations though the NCCN recommends tissue sampling if and when possible. See Chapter 2: Comprehensive Biomarker Testing.
Thoracic Tumor Board Diagnostic and Staging Algorithm
Today, most cancer centers have Tumor Boards which have been shown to improve the staging of cancer. Our center has an established Diagnostic Thoracic Tumor Board that brings together the knowledge and expertise of physicians from pulmonology, interventional pulmonology, medical oncology, radiation oncology, radiology, and thoracic surgery. To help us risk stratify lung lesions, we employ Lung RADS 1.1 for lung cancer screening detected lesions and Fleischner Society Guidelines 2017 for incidentally detected lung nodules.53-54 Patients with suspected or known lung cancer should receive rapid, cost-effective, accurate diagnosis and staging so that the appropriate treatment may be initiated in a timely manner. Our goal for all patients is to have a recommendation for management including a diagnosis and stage if lung cancer is suspected within 7 days of referral and to have the appropriate treatment initiated within 14 days.
All patients we evaluate with suspected or known NSCLC and who are potential candidates for surgical resection undergo PET-CT to evaluate for mediastinal disease and possible distant metastases. A prospective randomized trial evaluating the effect of combined PET-CT on the number of futile thoracotomies performed in patients with highly- suspected or newly diagnosed NSCLC.4-5 Futile thoracotomy was defined as a final diagnosis of a benign process, pathologically proven NSCLC stage IIIA-N2, IIIB, or IV disease, inoperable T3 or T4 disease, or recurrent malignancy or death from any cause within 1 year of randomization. A significant decrease in futile thoracotomies was achieved using PET-CT pre-operatively compared to conventional staging (21 of 60 vs. 38 of 73, p=0.05). A similar result was reported in an earlier publication using PET.55
Diagnosis if not previously made and staging is achieved by biopsy of the PET-avid lesion that would achieve the most advanced TNM stage. Biopsy methods for lymph nodes within the chest are described in Table 1. The preferred route of biopsy of mediastinal lymph nodes is to start with either EBUS or EUS depending upon the lymph node of interest. If the biopsy result is negative by EBUS or EUS, a confirmatory mediastinoscopy is recommended prior to proceeding to surgical resection.
Conclusion
Lung cancer survival is strongly associated with the stage of disease at the time of diagnosis and the resulting application of appropriate treatment. With the appropriate application of combined PET-CT, EBUS, EUS, and or mediastinoscopy, patients can now be accurately staged avoiding unnecessary surgery.
The reasons above are the reason our team believes so strongly in the importance of having a multidisciplinary panel of physicians to improve the timely application of appropriate staging and diagnostic studies.

Questions to Ask About Your Inital Diagnosis
Questions to Ask About Diagnostics
References
- Detterbeck FC. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Ccardiovasc Surg. 2018;155(1):356-9.
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. 2009 Jul;136(1):260-271. doi: 10.1378/chest.08-0978. PMID: 19584208.
- Sazon DA, Santiago SM, Soo Hoo GW, Khonsary A, Brown C, Mandelkern M, et al. Fluorodeoxyglucose-positron emission tomography in the detection and staging of lung cancer. American Journal of respiratory and critical care medicine. 1996;153(1):417-21.
- Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koeter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. The New England Journal of medicine. 2000;343(4):254-61.
- Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, et al. Preoperative staging of lung cancer with combined PET-CT. The New England Journal of medicine. 2009;361(1):32-9.
- Li X, Zhang H, Xing L, Ma H, Xie P, Zhang L, et al. Mediastinal lymph nodes staging by 18F-FDG PET/CT for early stage non-small cell lung cancer: a multicenter study. Radiotherapy and oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2012;102(2):246-50.
- Vella M, Meyer CS, Zhang N, Cohen BE, Whooley MA, Wang S, et al. Association of Receipt of Positron Emission Tomography-Computed Tomography With Non-Small Cell Lung Cancer Mortality in the Veterans Affairs Health Care System. JAMA Netw Open. 2019;2(11):e1915828.
- Garcia-Velloso MJ, Bastarrika G, de-Torres JP, Lozano MD, Sanchez-Salcedo P, Sancho L, et al. Assessment of indeterminate pulmonary nodules detected in lung cancer screening: Diagnostic accuracy of FDG PET/CT. Lung Cancer. 2016;97:81-6.
- Gao SJ, Kim AW, Puchalski JT, Bramley K, Detterbeck FC, Boffa DJ, et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer. 2017;109:36-41.
- Hjorthaug K, Højbjerg JA, Knap MM, Tietze A, Haraldsen A, Zacho HD, et al. Accuracy of 18F- FDG PET-CT in triaging lung cancer patients with suspected brain metastases for MRI. Nuclear Medicine Communications. 2015;36(11):1084-90.
- Hara T, Kosaka N, Suzuki T, Kudo K, Niino H. Uptake rates of 18F-fluorodeoxyglucose and 11C- choline in lung cancer and pulmonary tuberculosis: a positron emission tomography study. 2003;124(3):893-901.
- Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139(11):879-92.
- Erasmus JJ, McAdams HP, Patz EF, Jr., Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR American Journal of Roentgenology. 1998;170(5):1369-73.
- Cheran SK, Nielsen ND, Patz EF, Jr. False-negative findings for primary lung tumors on FDG positron emission tomography: staging and prognostic implications. AJR American Journal of Roentgenology. 2004;182(5):1129-32.
- Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van Tinteren H, Postmus PE, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. Journal of clinical oncology: official Journal of the American Society of Clinical Oncology. 2005;23(33):8362-70.
- Kremer R, Peysakhovich Y, Dan LF, Guralnik L, Kagna O, Nir RR, et al. FDG PET/CT for assessing the resectability of NSCLC patients with N2 disease after neoadjuvant therapy. Ann Nucl Med. 2016;30(2):114-21.
- Krasnik M, Vilmann P, Larsen SS, Jacobsen GK. Preliminary experience with a new method of endoscopic transbronchial real time ultrasound guided biopsy for diagnosis of mediastinal and hilar lesions. 2003;58(12):1083-6.
- Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, Iizasa T, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. 2004;126(1):122-8.
- Yasufuku K, Chiyo M, Koh E, Moriya Y, Iyoda A, Sekine Y, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50(3):347-54.
- Herth FJF, Eberhardt R, Becker HD, Ernst A. Endobronchial Ultrasound-Guided Transbronchial Lung Biopsy in Fluoroscopically Invisible Solitary Pulmonary Nodules: A Prospective Trial. 2006;129(1):147-50.
- Taverner J, Cheang MY, Antippa P, See K, Irving LB, Steinfort DP. Negative EBUS-TBNA Predicts Very Low Prevalence of Mediastinal Disease in Staging of Non-Small Cell Lung Cancer. J Bronchology Interv Pulmonol. 2016;23(2):177-80.
- Yarmus LB, Akulian J, Lechtzin N, Yasin F, Kamdar B, Ernst A, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: Results of the american college of chest physicians quality improvement registry, education, and evaluation registry. CHEST Journal. 2013;143(4):1036-43.
- Casal RF, Staerkel GA, Ost D, Almeida FA, Uzbeck MH, Eapen GA, et al. Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest. 2012;142(3):568-73.
- Scholten EL, Semaan R, Illei P, Mallow C, Arias S, Feller-Kopman D, et al. Stylet use does not improve diagnostic outcomes in endobronchial ultrasonographic transbronchial needle aspiration: A randomized clinical trial. Chest. 2017;151(3):636-42.
- Silvestri GA, Hoffman BJ, Bhutani MS, Hawes RH, Coppage L, Sanders-Cliette A, et al. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. The Annals of thoracic surgery. 1996;61(5):1441-5; discussion 5-6.
- Gress FG, Savides TJ, Sandler A, Kesler K, Conces D, Cummings O, et al. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: a comparison study. Ann Intern Med. 1997;127(8 Pt 1):604-12.
- Yarmus L, Akulian J, Wahidi M, Chen A, Steltz JP, Solomon SL, et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020;157(3):694-701.
- Chen AC, Pastis NJ, Jr., Mahajan AK, Khandhar SJ, Simoff MJ, Machuzak MS, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest. 2021;159(2):845-52.
- https://clinicaltrials.gov/ct2/show/NCT03893539 [last accessed 6/29/2021]
- https://clinicaltrials.gov/ct2/show/NCT04182815 [last accessed 6/29/2021]
- Ishida M, Suzuki M, Furumoto A, Tsuchihashi Y, Ariyoshi K, Morimoto K. Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath Increased the Diagnostic Yield of Peripheral Pulmonary Lesions. Internal medicine. 2012;51(5):455-60.
- Chen A, Chenna P, Loiselle A, Massoni J, Mayse M, Misselhorn D. Radial Probe Endobronchial Ultrasound for Peripheral Pulmonary Lesions. A 5-Year Institutional Experience. Annals of the American Thoracic Society. 2014;11(4):578-82.
- Sanchez-Font A, Giralt L, Vollmer I, Pijuan L, Gea J, Curull V. Endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions. A controlled study with fluoroscopy. Archivos de bronconeumologia. 2014;50(5):166-71.
- Okachi S, Imai N, Imaizumi K, Iwano S, Ando M, Hase T, et al. Factors Affecting the Diagnostic Yield of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in Peripheral Lung Cancer. Internal medicine. 2016;55(13):1705-12.
- Steinfort DP, Bonney A, See K, Irving LB. Sequential multimodality bronchoscopic investigation of peripheral pulmonary lesions. European Respiratory Journal. 2016;47(2):607-14.
- Sortini A, Navarra G, Santini M, Occhionorelli S, Sartori A, Bresadola V, et al. [Video-assisted mediastinoscopy. A new application of television technology in surgery]. Minerva chirurgica. 1994;49(9):803-5.
- Leschber G, Holinka G, Linder A. Video-assisted mediastinoscopic lymphadenectomy (VAMLA)–a method for systematic mediastinal lymphnode dissection. European Journal of cardio-thoracic surgery: official Journal of the European Association for Cardio-thoracic Surgery. 2003;24(2):192-5.
- Best LA, Munichor M, Ben-Shakhar M, Lemer J, Lichtig C, Peleg H. The contribution of anterior mediastinotomy in the diagnosis and evaluation of diseases of the mediastinum and lung. The Annals of thoracic surgery. 1987;43(1):78-81.
- Page A, Nakhle G, Mercier C, Verdant A, Page P, Dontigny L, et al. Surgical treatment of bronchogenic carcinoma: the importance of staging in evaluating late survival. Canadian Journal of surgery Journal canadien de chirurgie. 1987;30(2):96-9.
- Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA, American College of Chest P. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):202S-20S.
- Aleman C, Alegre J, Armadans L, Andreu J, Falco V, Recio J, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. The American Journal of medicine. 1999;107(4):340-3.
- Light RW. Clinical practice. Pleural effusion. The New England Journal of medicine. 2002;346(25):1971-7.
- Light RW. Pleural diseases. Current opinion in pulmonary medicine. 2003;9(4):251-3.
- Chen R-L, Zhang Y-Q, Wang J, Wu H, Yang S-M. Diagnostic value of medical thoracoscopy for undiagnosed pleural effusions. Experimental and therapeutic medicine. 2018;16(6):4590-4.
- Hansen M, Faurschou P, Clementsen P. Medical thoracoscopy, results and complications in 146 patients: a retrospective study. Respiratory medicine. 1998;92(2):228-32.
- Brims FJH, Arif M, Chauhan AJ. Outcomes and complications following medical thoracoscopy. The Clinical Respiratory Journal. 2012;6(3):144-9.
- Leemans J, Dooms C, Ninane V, Yserbyt J. Success rate of medical thoracoscopy and talc pleurodesis in malignant pleurisy: A single-centre experience. 2018;23(6):613-7.
- Chen J, Li Z, Xu N, Zhang X, Wang Y, Lin D. Efficacy of medical thoracoscopic talc pleurodesis in malignant pleural effusion caused by different types of tumors and different pathological classifications of lung cancer. Int J Clin Exp Med. 2015;8(10):18945-53.
- Landreneau RJ, Hazelrigg SR, Mack MJ, Fitzgibbon LD, Dowling RD, Acuff TE, et al. Thoracoscopic mediastinal lymph node sampling: useful for mediastinal lymph node stations inaccessible by cervical mediastinoscopy. The Journal of thoracic and cardiovascular surgery. 1993;106(3):554-8.
- Eggeling S, Martin T, Bottger J, Beinert T, Gellert K. Invasive staging of non-small cell lung cancer– a prospective study. European Journal of cardio-thoracic surgery: official Journal of the European Association for Cardio-thoracic Surgery. 2002;22(5):679-84.
- De Giacomo T, Rendina EA, Venuta F, Della Rocca G, Ricci C. Thoracoscopic staging of IIIB non- small cell lung cancer before neoadjuvant therapy. The Annals of thoracic surgery. 1997;64(5):1409-11.
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw. 2021;19(3):254-66.
- Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: Challenges and a Look Ahead, From the AJR Special Series on Radiology Reporting and Data Systems. American Journal of Roentgenology. 2021;216(6):1411-22.
- MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-43.
- Kubota K, Itoh M, Ozaki K, Ono S, Tashiro M, Yamaguchi K, et al. Advantage of delayed whole- body FDG-PET imaging for tumour detection. European Journal of nuclear medicine. 2001;28(6):696-703.

 Introduction
Introduction