Lung Cancer Choices© 6th Edition Menu
Chapter 1: Diagnosis and Staging of Lung Cancer
Chapter 2: Comprehensive Biomarker Testing
Chapter 3: Surgery for Lung Cancer Patients
Chapter 5: Radiation Therapy for Non-Small Cell Lung Cancer
Chapter 6: Treatment for Small Cell Lung Cancer
Chapter 7: Clinical Trials and Emerging Therapies for Lung Cancer
Chapter 9: Nutrition in the Patient with Lung Cancer
Chapter 10: Sexuality and Lung Cancer
Chapter 11: Integrative Medicine, Complementary Therapies, and Chinese Medicine in Lung Cancer
Chapter 12: Lung Cancer in People who have Never Smoked
Chapter 13: How to Quit Smoking Confidently and Successfully
Lung Cancer Question Builder - Develop your list of "Questions to Ask" your providers
Comprehensive Biomarker Testing
Kelly E. Goodwin, MSN, ANP-BC
Introduction
 Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer related deaths worldwide. Lung cancer rates vary significantly by sex, age, race/ethnicity, socioeconomic status, and geography, and lung cancer is found across all smoking histories.1 Lung cancer as a disease is as diverse as the patients diagnosed each year. Lung cancer is broadly classified as non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC). NSCLC represents approximately 85% of total lung cancer diagnoses, with adenocarcinoma (40%) and squamous cell carcinoma (25%) being the most commonly occurring histologies or subtypes.2 Curative and palliative therapies for lung cancer include intravenous or orally administered systemic therapies, radiation therapy, or surgery, and treatment is dependent on staging, pathology, and patient considerations.
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer related deaths worldwide. Lung cancer rates vary significantly by sex, age, race/ethnicity, socioeconomic status, and geography, and lung cancer is found across all smoking histories.1 Lung cancer as a disease is as diverse as the patients diagnosed each year. Lung cancer is broadly classified as non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC). NSCLC represents approximately 85% of total lung cancer diagnoses, with adenocarcinoma (40%) and squamous cell carcinoma (25%) being the most commonly occurring histologies or subtypes.2 Curative and palliative therapies for lung cancer include intravenous or orally administered systemic therapies, radiation therapy, or surgery, and treatment is dependent on staging, pathology, and patient considerations.
Systemic therapy for lung cancer historically consisted of chemotherapy. Chemotherapy works by non-specifically killing cells that are growing or dividing. While it can help improve quality of life and survival in some patients, it is also associated with significant toxicities due to the unintended effects on normal cells. Precision medicine, also called personalized medicine, is a medical model that aims to customize treatments based on individual variability in genes, environment, and lifestyle. Genes are portions of DNA that contain instructions for making the molecules that help our cells form and function. Through sequencing the genetic material of normal tissues and cancers, scientists have identified genetic alterations, often called mutations, that interfere with the normal functioning of cells. Some mutations change genes that control cell growth to be always active. Some other cancer-promoting mutations inactivate genes whose normal function is to slow or stop cell growth. Cells that have undergone oncogenic transformation due to a driver mutation are stuck in the “on” position, and their survival depends on a signal from that driver to survive. Reliance on, or addiction to, the oncogenic driver mutation makes certain lung cancers susceptible to targeted therapies. A better understanding of the molecular pathways that contribute to NSCLC and other malignancies since the early 2000s has led to the development of specific targeted therapies and immunotherapies that have helped to improve survival rates and decrease treatment-related toxicities for certain subsets of patients. More than a dozen new drugs for the treatment of NCSLC have been approved since 2013, marking an exciting and hopeful time in lung cancer research and care.3 With improved biomarker testing techniques, an expanding list of molecular targets, and more approved and emerging therapies, comprehensive biomarker testing is an essential part of the evaluation and management of all patients diagnosed with NSCLC.4
Biomarkers
Lung cancer biomarkers arise from somatic mutations and alterations in genes that occur in tissues. Somatic alterations differ from germline alterations in that they are not inherited from one’s parents and are not passed on to offspring. Somatic mutations are acquired after conception and may be due to environmental exposures or lifestyle choices (including smoking and poor diet) or through random errors during cell growth and division processes that turn DNA into RNA that encodes proteins. Somatic mutations can be found by testing tissue, blood, and fluids (like the pleural fluid around the lungs). Somatic mutations are classified as driver mutations when they encode proteins critical to cell growth, differentiation, and survival. Passenger mutations are those genetic variants that are less essential to transforming or maintaining the change of a noncancerous cell into a malignant cell.5 Germline mutations occur in the sperm or egg and at a very early age in fetal development such that they are presumed to be present in all of a person’s cells.
Germline alterations can increase one’s chances of developing cancer or may be associated with a family history of cancer called a predisposition syndrome. Germline mutations are typically identified through blood, saliva, or buccal (cheek) swabs, though they can be incidentally found during tumor testing.
Types of somatic genetic alterations include copy number alternations (extra gene copies or loss of gene copies), rearrangements or fusions (the abnormal juxtaposition of two genes that typically exist separately in the genetic material, effectively creating a new gene that is always “on”) and point mutations like deletions, insertions, substitutions or inversions (the word BEAST can be transformed to BEST with a deletion of “a”; to BREAST with the insertion of “r”; to FEAST with the substitution of “f”; or to BEATS with the inversion of the “s” and “t.”).
The American Society of Clinical Oncology (ASCO), the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), the Association for Molecular Pathology (AMP) and the National Comprehensive Cancer Network (NCCN) have issued guidelines for biomarker testing for patients with lung cancer since the early 2010s.6 Cancer promoting mutations in NSCLC were historically thought to occur in patients with a never or light smoking history and an adenocarcinoma histology and earlier versions of these guidelines reflected this understanding by recommending biomarker testing for specific target populations.7 Patient characteristics – including gender, age, ethnicity, smoking history, frailty, burden of disease and insurance coverage – as well as access to testing, insufficient tissue (due to sampling technique or exhausted in earlier diagnostic testing), long turnaround times, healthcare spending concerns and lack of knowledge of actionable mutations among providers have historically been barriers to biomarker testing.8 Studies have overwhelmingly shown improved overall survival for patients who have undergone biomarker testing and for those who have biomarker testing results available prior to the start of first line therapy.9 This survival benefit is due in part to improved adherence to guideline recommendations for targeted agents when an actionable mutation exists, avoidance of regimens with decreased efficacy in the presence of certain mutations and a more thoughtful approach to the sequencing of available therapies. Current guidelines now recommend comprehensive molecular genotyping before initiating treatment for all patients with newly diagnosed advanced NSCLC.10
Biomarker Testing Methods
Tissue-based testing has historically been the gold standard for molecular analysis of NSCLC. Because driver mutations develop early in the process of transformation from a normal cell to a malignant cell, tumor samples from either the primary tumor or a metastatic lesion are appropriate for molecular testing.12 Once the tissue has been procured through a diagnostic biopsy, confirmation of adequate tumor cellularity occurs, and histology has been determined (squamous versus nonsquamous), the tissue sample may undergo biomarker testing. “Liquid biopsies” detect fragments of circulating tumor DNA (ctDNA) that are shed into the bloodstream or other fluids when the cancer is most active (at the time of diagnosis or when cancer is progressing on a therapy). Until recently, liquid biopsies were used primarily for inaccessible tumors or when a tissue biopsy (or repeat biopsy in the case of an insufficient sample) was considered too high risk. ctDNA testing has significant benefits – it is less invasive than a traditional tissue biopsy, quick (7-10 days), and easily repeatable. The sensitivity of detecting target mutations with ctDNA is 60-80% and depends on tumor location, size, blood supply, and the detection method used (PCR vs. NGS).13 Because of the increased risk of false negative ctDNA tests, liquid biopsies do not serve as stand-alone testing – tissue-based testing should be considered if clinical suspicion for an activating mutation is high. Recent studies have shown that plasma ctDNA testing is non-inferior to tissue-based testing and that plasma testing conducted at the same time or even before tissue-based testing in a subset of patients with radiographic findings concerning advanced lung cancer can decrease molecular genotyping turnaround time, improve identification of actionable targets and accelerate the time to treatment for all patients without adding increased costs.14
Clinically useful biomarker tests can be performed on available samples, are at least semi-automated, do not rely on a single operator or interpreter, are cost-effective, and have a fast turnaround time (two weeks or less).15 The type of testing employed depends on the kind of mutation under investigation – DNA mutations, chromosome abnormalities, or gene expression.
The most common testing techniques include but are not limited to, polymerase chain reaction testing (PCR) of DNA or RNA (the genetic material that converts DNA into proteins), Fluorescence in-situ testing (FISH), Immunohistochemistry (IHC) and Next Generation Sequencing (NGS). These tests are collectively called companion diagnostics because they help match a patient to a specific drug or therapy. Testing can be performed through a hospital based, accredited laboratory or several commercial services.
DNA sequencing and allele-specific testing are forms of single gene tests that use PCR to examine the entire length of a gene or a specific region of a chromosome for the presence of a pre-specified mutation. While a relatively quick turnaround time of less than one week is an advantage to these testing techniques, there are several disadvantages, including lower sensitivity (a higher probability of a false negative), the potential to exhaust the tissue sample through multiple single gene tests, low-cost effectiveness and the inability to identify new abnormalities.16
Fluorescence in-situ testing (FISH) is a testing technique that detects gene rearrangements, translocations, amplifications, or deletions by visualizing and mapping the genetic materials in cells. FISH is useful for examining specific genes or portions of genes or understanding larger chromosomal abnormalities. Short sequences of single-stranded DNA called “probes” are created to match a portion of the gene under evaluation; each probe is “labeled” with a fluorescent dye. As DNA is double stranded, identifying rearrangements uses hybridizing DNA probes of different colors that separate when two parts of a gene have broken apart.17 FISH testing can only detect a pre-specified mutation, requires significant technical expertise to perform and interpret and requires a minimum of 50-100 well preserved tumor cells within the tissue sample. FISH results are typically available in about six days.18
Immunohistochemistry (IHC) testing is used to detect the presence of specific proteins in cells or tissues that may be overexpressed, whether or not there is an alteration in the genes. This technique preserves the spatial context or architecture of the tissue sample. The role of IHC testing in NSCLC continues to evolve, but it is a rapid (one day) and reliable test for several of the most common driver mutations found in NSCLC.19
Next-generation sequencing (NGS) is a broad-based testing technique that allows for the analysis of multiple genetic alterations at the same time. DNA fragments from the biopsied sample are purified, amplified, isolated, and compared to a known mutation “library” and a normal reference sample. Several hospital-based and commercially targeted lung cancer NGS tests are available that can test for 10s-100 alterations.20 Single gene testing requires “purer” cancer samples for adequate sensitivity and can exhaust tissue when run sequentially. NGS can be financially expensive though more cost effective than a la carte testing, and the median turnaround time for results is about 2-3 weeks. Rapid and ultra-rapid testing of some of the most common mutation types can be added as an adjunct to NGS testing for patients with significant symptoms and certain pathologic or clinical features. These rapid or ultra-rapid testing pathways can return results in 9 days or less than two days respectively and can significantly shorten the time between diagnosis and initiation of a life sustaining therapy.21 NGS reports can contain a large amount of information and require careful interpretation before finalizing a treatment plan. Acknowledging the importance of comprehensive biomarker testing for patients with recurrent, metastatic, refractory, or stages III or IV cancer, the Centers for Medicare and Medicaid finalized a National Coverage Determination in 2018 that covers diagnostic laboratory tests using NGS to assist oncologists and patients in making more appropriate and timely treatment decisions and determining candidacy for clinical trials.22 Private payor reimbursement is variable but many oncologists, pathology departments and commercial labs have processes to advocate for reduced out of pocket costs to the patient.
Lung Cancer Biomarkers
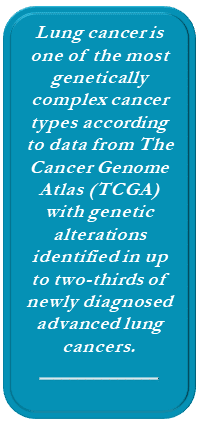 Lung cancer is one of the most genetically complex cancer types, according to data from The Cancer Genome Atlas (TCGA), with genetic alterations identified in up to two-thirds of newly diagnosed advanced lung cancers. Historically driver mutations have been thought to occur primarily in nonsquamous tumors and to be mutually exclusive.23 However, recent studies have shown clinically significant, actionable mutations in approximately 10% of squamous cell tumors24 and overlapping driver mutations in up to 12% of nonsquamous NSCLC.25 Coexistence of driver mutations is clinically relevant because it may provide targets for additional or combination therapy and may help to identify potential sensitivity or resistance to a particular type of targeted therapy.
Lung cancer is one of the most genetically complex cancer types, according to data from The Cancer Genome Atlas (TCGA), with genetic alterations identified in up to two-thirds of newly diagnosed advanced lung cancers. Historically driver mutations have been thought to occur primarily in nonsquamous tumors and to be mutually exclusive.23 However, recent studies have shown clinically significant, actionable mutations in approximately 10% of squamous cell tumors24 and overlapping driver mutations in up to 12% of nonsquamous NSCLC.25 Coexistence of driver mutations is clinically relevant because it may provide targets for additional or combination therapy and may help to identify potential sensitivity or resistance to a particular type of targeted therapy.
In 2020 and 2021, the NCCN updated their guidelines for routine molecular testing in newly diagnosed NSCLC, recommending testing be performed via a broad, panel-based approach, most typically performed by NGS, so that testing is done for all of the actionable biomarkers at the same time, including the established and emerging biomarkers. At a minimum, testing results for EGFR mutations, ALK rearrangements, ROS1 fusions, BRAF V600E mutations, RET fusions, MET exon 14 skipping mutations, NTRK fusions, KRAS mutations, and PD-L1 protein overexpression should be available before initiating first-line therapy in patients with nonsquamous histologies. Testing should be considered for squamous cell histology.26 The expert panel recommended that smoking status, small biopsy specimens, and mixed histology should no longer be used when considering whether to perform biomarker testing. Furthermore, while acknowledging the lower incidence of driver mutations in advanced squamous cell NSCLC, the panel cited the cumulative incidence of actional alternations in squamous cell tumors and the effectiveness of targeted therapies as justification for recommending comprehensive biomarker testing for tumors of squamous histology. The goal of these expanded guidelines is to identify rarer mutations for which effective drugs may be available and to identify patients for appropriate clinical trials to advance the care of all patients with NSCLC.27
Common biomarkers in NSCLC
EGFR (Epidermal Growth Factor Receptor) mutations are the most common targetable mutations found in lung adenocarcinomas, accounting for nearly 20% of NSCLC, and are often found in younger patients, Asian patients, and patients with a never or light smoking history. EGFR mutations can be detected with PCR or NGS testing. EGFR exon 19 deletions, L858R point mutations and some exon 19 insertions are associated with responsiveness to multiple oral EGFR targeted therapies. Targeted therapies for these EGFR mutations have been less effective for EGFR exon20 insertion mutant NSCLC, which represents about 10% of EGFR mutations and only 2% of overall lung adenocarcinomas, but two new therapeutic options to be used after initial treatment with chemotherapy (one oral and one administered by infusion) have recently been authorized.28
ALK (Anaplastic Lymphoma Kinase) gene rearrangements have been found in 3-5% of patients with NSCLC and can be found using FISH, IHC, and numerous NGS methods. ALK rearrangements are more frequently found in younger males, never-smokers with adenocarcinoma histology, and ALK positive disease is associated with responsiveness to multiple oral ALK inhibitors.29
ROS1 (ROS proto-oncogene 1) rearrangements, typically genetic translocations, have been identified by FISH or NGS, though some variants may be underreported. ROS1 mutations act as the driver mutation in 1-2% of NSCLCs and are more frequently identified in younger Asian, never-smokers with adenocarcinoma histology. The presence of ROS1 rearrangement predicts responsiveness to oral ROS1 targeted therapies.30
BRAF (B-raf proto-oncogene) mutations, detected through PCR and NGS methods, are found in up to 4% of NSCLC, though only the V600E variant has been associated with responses to BRAF inhibition given in combination with another class of drugs targeting the MEK pathway. There are currently two approved oral combinations of BRAF + MEK inhibition. BRAF mutations are most commonly found in patients with a smoking history.31
MET (mesenchymal-epithelial transition) mutations in lung cancer exist as structural mutations (exon 14 skipping mutations) or functional mutations (MET gene overexpression/amplifications) and are found in approximately 2-4% of NSCLC through RNA based NGS testing.
Variants in MET are associated with response to oral therapies that inhibit MET. There are currently 2 MET inhibitors available in the US. MET exon 14 skipping mutations are more frequently identified in patients with nonsquamous tumor histology, older, female, and are less likely to be non-smokers.32
RET (rearranged during transfection) rearrangements are detected by FISH, RNA-based NGS, or PCR testing in 1-2% of lung adenocarcinomas and are associated with responses to oral RET inhibitors. RET rearrangement occurs more frequently in younger patients and in never-smokers. There are currently two approved RET inhibitors on the market.33
KRAS (Kirsten rat sarcoma) mutations are found in approximately 25% of patients with lung adenocarcinoma and are generally associated with smoking. KRAS G12C is the most common KRAS mutation found in lung cancers and is responsible for approximately 1 in every eight lung adenocarcinomas. Two KRAS G12C inhibitors have been approved for the treatment of disease following progression on or intolerance of first line treatment for advanced disease.34
NTRK 1/2/3 (neurotrophic tyrosine receptor kinase) gene fusions have been found in approximately 1% of NSCLC via FISH, IHC, PCR, and NGS testing and are associated with sensitivity to oral NTRK inhibitors. Two therapies active against NTRK are currently available for use in combination with chemotherapy in the initial treatment of the disease or following the progression or intolerance of chemotherapy. NTRK fusions occur in NSCLCs across sexes, ages, smoking histories, and histologies.35
PD-L1 (programmed death-ligand 1) is a protein found on cancer cells, including NSCLC, which helps the cancer to hide from the immune system. PD-L1 expression can be detected using IHC and is reported as the proportion of tumor cells exhibiting staining. The proportion of PD-L1 informs the decision to pursue immunotherapy alone (PD-L1>50%) or chemotherapy plus immunotherapy combinations (PD-L1>1%) in patients with newly diagnosed NSCLC.36 Multiple immunotherapies are approved for use in NSCLC across stages. Patients with EGFR and ALK alterations rarely respond to immunotherapy even in the presence of high PD-L1 expression and should be treated with targeted therapy.37 TMB (tumor mutational burden) is not currently recommended for NSCLC but is emerging as an independent predictor of response to immunotherapy.38
HER2 (human epidermal growth factor receptor 2) mutations have been reported in 1-3% of NSCLC tumors, are most frequently detected using PCR or NSG testing, and predominantly never affect smokers. The majority of HER2 mutations occur in women with adenocarcinomas.
Anti-HER2 therapies are currently approved for HER2 mutations or overexpression in other cancer types (breast cancer and gastrointestinal cancers). One therapy is now approved for use following disease progression on standard first line chemotherapy or chemotherapy with immunotherapy. Additional agents are in development and may be accessed through clinical trial participation.39
Additional emerging biomarkers include PTEN, FGFR1, PDGFRA and DDR2. These alterations are most commonly found in squamous cell lung cancers. Multiple therapies directed at these variations are currently in development.40
Conclusion
As our understanding of genetic drivers in NSCLC evolves, and more therapeutic options become available, patients are living longer and challenging the traditional concept of cancer survivorship. A collaborative, multidisciplinary approach to the evaluation and management of NSCLC that utilizes comprehensive biomarker testing for all patients with newly diagnosed NSCLC regardless of age, gender, smoking history, or histology and for patients with identified actionable mutations whose cancer is acquiring resistance to targeted therapies is critical to ensuring that patients receive the therapy that is most likely to improve their survival and quality of life.41

Questions to Ask About Diagnostics
References
- Goodwin K.E., Davies M. (2019) Nursing Considerations for Patients Treated with Targeted Therapies. In: Davies M., Eaby-Sandy B. (eds) Targeted Therapies in Lung Cancer: Management Strategies for Nurses and Practitioners. Springer, Cham. https://doi.org/10.1007/978-3-030-16550-5_9
- Travis WD, Brambilla E, Nicholson AG, et al. WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015 Sep;10(9):1243-1260.
- Stein J, Mann J. Specialty pharmacy services for patients receiving oral medications for solid tumors. Am J Health Syst Pharm. 2016;73(11):775–96.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2021). https://www.nccn.org/progressionals/physician_gls/pdf/nscl.pdf. Accessed 6/1/2021.
- Sequist LV, Neal JW. Personalized, genotype-directed therapy for advanced non-small cell lung cancer. In UpToDate, Lilenbaum RC (Ed), UpToDate, Waltham, MA. (Accessed on June 15, 2021.)
- Pennell NA, Arcila ME, Gandara DR, West H. Biomarker Testing for Patients With Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am Soc Clin Oncol Educ Book. 2019 Jan;39:531-542. doi: 10.1200/EDBK_237863. Epub 2019 May 17. PMID: 31099633.
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cel lung cancer who harbor EML4-ALK. J Clin Oncol 2009; 27: 4247-53
- Aggarwal C, Marmarelis ME, Hwang WT, et al. Association Between Availability of Molecular Genotyping Results and Overall Survival in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer. JCO Precis Oncol. 2023 Jul;7:e2300191. doi: 10.1200/PO.23.00191. PMID: 37499192.
- John A, Shah RA, Wong WB, et al. Value of precision medicine in advanced non-small cell lung cancer. Real-world outcomes associated associated with the use of companion diagnostics. Oncologist. 2020; 25:e1743-e1752.
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. The Lancet 2017; 389: 299-311
- Audibert CM, Shea MB, Glass DJ, et al. Trends in the Molecular Diagnosis of Lung Cancer: Results from an Online Market Research Survey. Washington, DC: Friends of Cancer Research; 2016. https://friendsofcancerresearch.org/sites/default/files/FINAL%202017%20Friends%20NSCLC%20White%20Paper_0.pdf. Accessed July 1, 2021.
- Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011; 29: 2972-7.
- Sacher AG, Paweletz C, Dahlbger SE, et al. Prospective validation of rapid plasma genotyping in advanced lung cancer. JAMA Oncol 2016; 2: 1014-22.
- Garcia-Pardo M, Czarnecka-Kujawa K, Law JH, et al. Association of circulating tumor DNA testing before tissue diagnosis with time to treatment among patients with suspected advanced lung cancer. The ACCELERATE nonrandomized clinical trial. JAMA Netw Open. 2023 Jul 3;6(7):e2325332. doi: 10.1001/jamanetworkopen.2023.25332. PMID: 37490292; PMCID: PMC10369925.
- Sequist LV, Neal JW. Personalized, genotype-directed therapy for advanced non-small cell lung cancer. In UpToDate, Lilenbaum RC (Ed), UpToDate, Waltham, MA. (Accessed on June 15, 2021.)
- Ellison G, Zhu G, Moulis A, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology. J Clin Pathol. 2013; 66:79-89.
- Tang Z, Wang L, Tang G, Medeiros LJ. Fluorescence in Situ Hybridization (FISH) for Detecting Anaplastic Lymphoma Kinase (ALK) Rearrangement in Lung Cancer: Clinically Relevant Technical Aspects. Int J Mol Sci 2019; 20(16):3939.
- Illei PB, Wong W, Wu N, et al. ALK Testing Trends and Patterns Among Community Practices in the Unites States. JCO Prec Oncol 2018; 2: 1-11.
- Sequist LV, Neal JW. Personalized, genotype-directed therapy for advanced non-small cell lung cancer. In UpToDate, Lilenbaum RC (Ed), UpToDate, Waltham, MA. (Accessed on June 15, 2021.)
- Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011 Dec;22(12):2616-2624.
- Dagogo-Jack I, Azzolli CG, Fintelmann F, et al. Clinical Utility of Rapid EGFR Genotyping in Advanced Lung Cancer. JCO Precis Oncol. 2018;2018:PO.17.00299.
- Centers for Medicare & Medicaid Services. Decision Memo for Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer (CAG-00450N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=209. Accessed July 14, 2021
- Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC). J Clin Oncol. 2011;29:18s, CRA7506-CRA7506.
- Lam VK, Tran HT, Banks KC, et al. Targeted tissue and cell-free tumor DNA sequencing of advanced lung squamous-cell carcinoma reveals clinically significant prevalence of actionable alterations. Clin Lung Cancer 2019;20:30–36.e3
- Martín Martorell P, Huerta M, Compañ Quilis A, et al. Coexistence of EGFR, KRAS, BRAF, and PIK3CA Mutations and ALK Rearrangement in a Comprehensive Cohort of 326 Consecutive Spanish Nonsquamous NSCLC Patients. Clin Lung Cancer. 2017 Nov;18(6):e395-e402.
- Robert NJ, Espirito JL, Chen L, et al. Biomarker testing and tissue journey among patients with metastatic non-small cell lung cancer receiving first-line therapy in The US Oncology Network. Lung Cancer. 2022 Apr;166:197-204. doi: 10.1016/j.lungcan.2022.03.004. Epub 2022 Mar 10. PMID: 35313244.
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021, Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 19(3), 254-266.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2021). https://www.nccn.org/progressionals/physician_gls/pdf/nscl.pdf. Accessed 6/1/2021.
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009; 27: 4247-53.
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014; 371: 1963-71.
- Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 2015; 121:448.
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol. 2016 Mar 1;34(7):721-30.
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012; 18:378.
- Sotorasib tablets. United States Prescribing Information. US National Library of Medicine. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214665s000lbl.pdf. Accessed July 10, 2021.
- Farago AF, Taylor MS, Doebele RC, et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis Oncol. 2018;2018:PO.18.00037.
- Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell SF, Cheng SY, Bischoff HG, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2020 May 10;38(14):1505-1517.
- Aggarwal C, Marmarelis ME, Hwang WT, et al. Association Between Availability of Molecular Genotyping Results and Overall Survival in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer. JCO Precis Oncol. 2023 Jul;7:e2300191. doi: 10.1200/PO.23.00191. PMID: 37499192.
- Riszvi NA, Hellman MD, Snyder A, et al. Cancer Immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124-128.
- Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer. 2017 Nov 1;123(21):4099-4105.
- Heist RS, Engelman JA. SnapShot: Non-Small Cell Lung Cancer. Cancer Cell 2012; 21:448.
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancer to select targeted drugs. JAMA 2014; 311:1998.